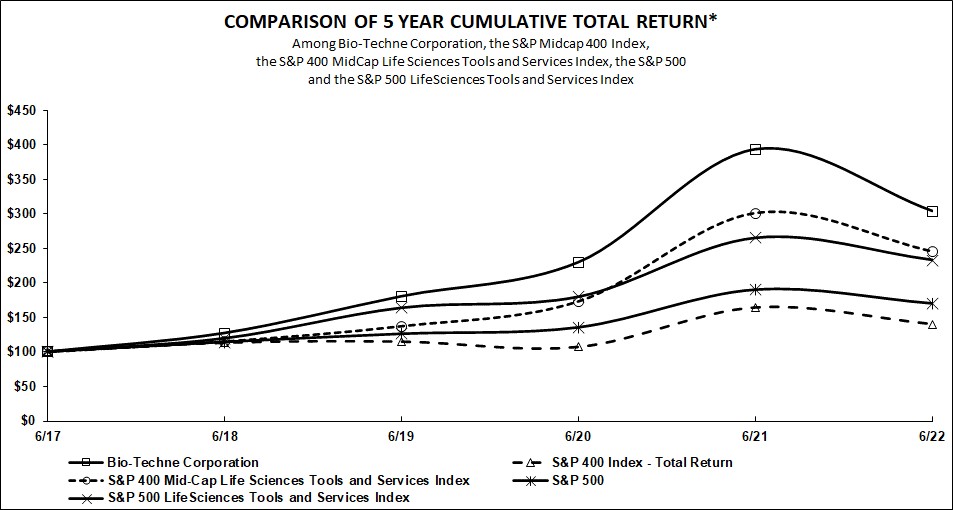
Company became part of the S&P 500 Index during fiscal 2022. The S&P 400 Index was included for comparative purposes to the prior year Form 10-K.
31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the fiscal year ended | |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period
from to
Commission file number
(Exact name of registrant as specified in its charter)
| ||
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification No.) |
|
| |
| ( | |
(Address of principal executive offices) (Zip Code) |
| (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| ☒ | Accelerated filer | ☐ |
|
|
|
|
Non-accelerated filer | ☐ | Smaller reporting company | |
|
|
|
|
|
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 USC. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. Yes
As of December 31, 2021, the aggregate market value of the Common Stock held by non-affiliates of the Registrant was $
As of August 19, 2022,
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Company’s Proxy Statement for its 2022 Annual Meeting of Shareholders are incorporated by reference into Part III.
TABLE OF CONTENTS
2
In this Annual Report, the terms “Bio-Techne” or the “Company” refer to Bio-Techne Corporation, Bio-Techne Corporation and its consolidated subsidiaries, or the consolidated subsidiaries of Bio-Techne Corporation, as the context requires.
FORWARD-LOOKING INFORMATION AND CAUTIONARY STATEMENTS
Certain statements included or incorporated by reference in this Annual Report, in other documents we file with or furnish to the Securities and Exchange Commission (“SEC”), in our press releases, webcasts, conference calls, materials delivered to shareholders and other communications, are “forward-looking statements” within the meaning of the U.S. federal securities laws. All statements other than historical factual information are forward-looking statements, including without limitation statements regarding: projections of revenue, expenses, profit, profit margins, pricing, tax rates, tax provisions, cash flows, our liquidity position or other projected financial measures; management’s plans and strategies for future operations, including statements relating to anticipated operating performance, cost reductions, new product and service developments, competitive strengths or market position, acquisitions and the integration thereof, strategic opportunities, dividends and executive compensation; growth, declines and other trends in markets we sell into; new or modified laws, regulations and accounting pronouncements; future regulatory approvals and the timing and conditionality thereof; outstanding claims, legal proceedings, tax audits and assessments and other contingent liabilities; future foreign currency exchange rates and fluctuations in those rates; the potential or anticipated direct or indirect impact of COVID-19 on our business, results of operations and/or financial condition; general economic and capital markets conditions; the anticipated timing of any of the foregoing; assumptions underlying any of the foregoing; and any other statements that address events or developments that Bio-Techne intends or believes will or may occur in the future. Terminology such as “believe,” “anticipate,” “should,” “could,” “intend,” “will,” “plan,” “expect,” “estimate,” “project,” “target,” “may,” “possible,” “potential,” “forecast” and “positioned” and similar references to future periods are intended to identify forward-looking statements, although not all forward-looking statements are accompanied by such words. Forward-looking statements are based on assumptions and assessments made by our management in light of their experience and perceptions of historical trends, current conditions, expected future developments and other factors they believe to be appropriate. These forward-looking statements are subject to a number of risks and uncertainties, including but not limited to the risks and uncertainties set forth below and under “Item 1A. Risk Factors” in this Annual Report.
Forward-looking statements are not guaranties of future performance and actual results may differ materially from the results, developments and business decisions contemplated by our forward-looking statements. Accordingly, you should not place undue reliance on any such forward-looking statements. Forward-looking statements speak only as of the date of the report, document, press release, webcast, call, materials or other communication in which they are made. Except to the extent required by applicable law, we do not assume any obligation to update or revise any forward-looking statement, whether as a result of new information, future events and developments or otherwise.
Investment in our securities involves risk and uncertainty and you should carefully consider all information in this Annual Report on Form 10-K prior to making an investment decision regarding our securities. Below is a summary of material risks and uncertainties we face, which are discussed more fully in “Item 1A. Risk Factors”:
Business and Strategic Risks
| ● | Conditions in the global economy, the particular markets we serve and the financial markets, whether brought about by material global crises or other factors, may adversely affect our business and financial results. |
| ● | International political, compliance and business factors, including the military conflict in Ukraine and the United Kingdom’s withdrawal from the European Union, can negatively impact our operations and financial results. |
| ● | The healthcare and life sciences industries that we serve face constant pressures and changes in an effort to reduce healthcare costs or increase their predictability, all of which may adversely affect our business and financial results. |
3
Acquisition and Investment Risks
Operational Risks
| ● | If we cannot adjust our manufacturing capacity or purchases required for our manufacturing activities to reflect changes in market conditions or customer demand, our business and financial results may suffer. In addition, our reliance upon sole or limited sources of supply for certain materials, components and services can cause production interruptions, delays and inefficiencies. |
| ● | Climate change, or legal or regulatory measures to address climate change, may negatively affect us. |
4
Intellectual Property Risks
Financial and Tax Risks
| ● | Our business and financial results can be adversely affected by foreign currency exchange rates, changes in our tax rates, and tax liabilities and assessments (including as a result of changes in tax laws). |
Legal, Regulatory, Compliance and Reputational Risks
| ● | Our business is subject to extensive regulation; failure to comply with these regulations could adversely affect our business and financial results. |
5
PART I
ITEM 1. BUSINESS
OVERVIEW
Bio-Techne and its subsidiaries, collectively doing business as Bio-Techne Corporation (Bio-Techne, we, our, us or the Company), develop, manufacture and sell life science reagents, instruments and services for the research, diagnostics and bioprocessing markets worldwide. With our broad product portfolio and application expertise, we sell integral components of scientific investigations into biological processes and molecular diagnostics, revealing the nature, diagnosis, etiology and progression of specific diseases. Our products aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses.
We manage the business in two operating segments – our Protein Sciences segment and our Diagnostics and Genomics segment. Our Protein Sciences segment is a leading developer and manufacturer of high-quality biological reagents used in all aspects of life science research, diagnostics and cell and gene therapy. This segment also includes proteomic analytical tools, both manual and automated, that offer researchers and pharmaceutical manufacturers efficient and streamlined options for protein size and purity analysis, automated western blot and multiplexed ELISA workflow. Our Diagnostics and Genomics segment develops and manufactures diagnostic products, including controls, calibrators, and diagnostic assays for the regulated diagnostics market, exosome-based molecular diagnostic assays, advanced tissue-based in-situ hybridization assays for spatial genomic and tissue biopsy analysis, and genetic and oncology kits for research and clinical applications.
We are a Minnesota corporation with our global headquarters in Minneapolis, Minnesota. We were founded in 1976 as Research and Diagnostic Systems, Inc. We became a publicly traded company in 1985 through a merger with Techne Corporation, now Bio-Techne Corporation. Our common stock is listed on the NASDAQ under the symbol “TECH.” We operate globally, with offices in many locations throughout North America, Europe and Asia. Today, our product lines include hundreds of thousands of diverse products, most of which we manufacture ourselves in multiple locations in North America, as well as a location each in the U.K. and China.
Our historical focus was on providing high quality proteins, antibodies and immunoassays to the life science research market and hematology controls to the diagnostics market. Over the last ten years, we have been implementing a disciplined strategy to accelerate growth and expand our addressable markets in part by acquiring businesses and product portfolios that leveraged and diversified our existing product lines, filled portfolio gaps with differentiated high growth businesses, and expanded our geographic scope. From fiscal years 2013 through 2022 we have acquired sixteen companies that have expanded the product offerings and geographic footprint of both operating segments. Recognizing the importance of an integrated, global approach to meeting our mission and accomplishing our strategies, we have maintained many of the brands of the companies we have acquired, but unified under a single global brand -- Bio-Techne.
We are committed to providing the life sciences community with innovative, high-quality scientific tools that allow our customers to make extraordinary discoveries and diagnose diseases. We intend to build on Bio-Techne’s past accomplishments, high product quality reputation and sound financial position by executing strategies that position us to serve as the standard for biological content in the research market, and to leverage that leadership position to enter the diagnostics and other adjacent markets. Our strategies, which have been consistent for at least the last several years, include:
Continued innovation in core products. Through collaborations with key opinion leaders, participation in scientific discussions and societies, and leveraging our internal talent we expect to be able to convert our continued significant investment in our research and development activities to be first-to-market with quality products that are at the leading edge of life science researchers’ needs.
Market and geographic expansion. We will continue to expand our sales staff and distribution channels globally in order to increase our global presence and make it easier for customers to transact with us. We will also leverage our existing portfolio to expand our product offerings into novel research fields and further into diagnostics and therapeutics markets.
6
Culture development and talent recruitment and retention. As we continue to grow both organically and through acquisition, we are intentionally fostering an “EPIC” culture based on the ideals of Empowerment, Passion, Innovation and Collaboration. We strive to recruit, train and retain the most talented staff, who share these EPIC ideals to effectively implement our global strategies.
Targeted acquisitions and investments. We will continue to leverage our strong balance sheet to gain access to new and differentiated technologies and products that improve our competitiveness in the current market, meet customers’ expanding workflow needs and allow us to enter adjacent markets.
PROTEIN SCIENCES SEGMENT
Protein Sciences Segment Products and Markets
The Protein Sciences segment is the larger of our two segments, representing about 75% of our net sales in fiscal 2022. It is comprised of two divisions with complementary product offerings serving many of the same customers – the Reagent Solutions division and the Analytical Solutions division.
The Reagent Solutions division consists of specialized proteins, such as cytokines and growth factors, antibodies, small molecules, tissue culture sera and cell selection technologies traditionally used by researchers to further their life science experimental activities and by companies developing next generation diagnostics and therapeutics, including companies developing cell- and gene-based therapeutics. We believe we are the world leader in providing high quality proteins, both for research use and under current Good Manufacturing Practices, or cGMP. Key product brands include R&D Systems, Tocris Biosciences and Novus Biologicals. Our combined chemical and biological reagents portfolio provides high quality tools that customers can use in solving complex biological pathways and glean knowledge that may lead to a more complete understanding of biological processes, and, ultimately, to the development of novel therapeutic strategies to address different pathologies. In recent years, we have made several acquisitions and investments that have expanded our product offerings for the cell and gene therapy market. These include a significant investment in state-of-the art facilities for production of both proteins and small molecules in large quantities manufactured in accordance with cGMP, as well as an agreement entered into in fiscal 2022 to invest in and potentially acquire Wilson Wolf Manufacturing Company, which is a leading provider of cell culture devices for cell therapy. Through a collaborative marketing venture with Wilson Wolf and another company, we have leveraged products we have or are developing to provide a more complete offering for the cell and gene therapy market.
The Analytical Solutions division includes manual and automated protein analysis instruments and immunoassays that are used in quantifying proteins in a variety of biological fluids. Products in this division include traditional manual plate-based immunoassays, fully automated multiplex immunoassays on various instrument platforms, automated western blotting and isoelectric focusing analysis of complex protein samples. Key product brands include R&D Systems and ProteinSimple. A number of our products have been demonstrated to have the potential to serve as predictive biomarkers and therapeutic targets for a variety of human diseases and conditions including cancer, autoimmunity, diabetes, hypertension, obesity, inflammation, neurological disorders, and kidney failure. Immunoassays can also be useful in clinical diagnostics. In fact, we have received Food and Drug Administration (FDA) marketing clearance for a few of our immunoassays for use as in vitro diagnostic devices. In addition, subsequent to fiscal 2022, we closed on the acquisition of Namocell, Inc., a leading provider of single cell sorting and dispensing platforms that are gentle to cells and therefore preserve cell viability and integrity.
Protein Sciences Segment Customers and Distribution Methods
Our customers for this segment include researchers in academia, government and industry (chiefly pharmaceutical and biotech companies as well as contract research organizations). This segment also sells to diagnostic/companion diagnostic and therapeutic customers, including customers engaged in the development of cell- and gene-based therapies. Our biologics line of products in the Analytical Solutions division is used chiefly by production and quality control departments at biotech and pharmaceutical companies. We sell our products directly to customers who are primarily located in North America, Europe and China, as well as through a distribution agreement with Fisher Scientific. We also sell through third party distributors in China, Japan, certain eastern European countries and the rest of the world. Our sales are widely
7
distributed, and no single end-user customer accounted for more than 10% of the Protein Sciences segment’s net sales during fiscal 2022, 2021 and 2020.
DIAGNOSTICS AND GENOMICS SEGMENT
The Diagnostics and Genomics segment, representing about 25% of our net revenues in fiscal 2022, is comprised of three divisions and is focused primarily on the diagnostics market and includes spatial biology, liquid biopsy, molecular diagnostics kits and products, and diagnostics reagents.
Diagnostics and Genomics Segment Products
The Spatial Biology division products sold under the Advanced Cell Diagnostics, or ACD, brand, are novel in-situ hybridization (ISH) assays for transcriptome, DNA copy, and structural variation analysis within intact cells, providing highly sensitive and specific spatial information at single cell resolution. Since these products preserve spatial context, they are particularly useful for complex tissue profiling.
The Molecular Diagnostics division markets and sells products and services under the Exosome Diagnostics and Asuragen brands. The Exosome Diagnostics brand is based on exosome-based liquid biopsy techniques that analyze genes or their transcripts. It includes the ExoDx Prostate test, which is a urine-based assay for early detection of high-grade prostate cancer used as an aid in deciding the need for biopsy and offered by Exosome Diagnostics as a lab-developed test, as well as the ExoTRU kidney transplant rejection test, which we have licensed exclusively to Thermo Fisher Scientific. We also sell products for genetic carrier screening, oncology diagnostics, molecular controls, and research under the Asuragen brand.
The Diagnostic Reagents division consists of regulated products traditionally used as calibrators and controls in the clinical setting. Also included are instrument and process control products for hematology, blood chemistry, blood gases, coagulation controls and reagents used in various diagnostic applications. We often manufacture these reagents on a custom basis, tailored to a customer’s specific diagnostic assay technology. We supply these reagents in various formats including liquid, frozen, or in lyophilized form. Most of these products are sold on an Original Equipment Manufacturer (OEM) basis to instrument manufacturers, with most products being FDA-cleared.
Diagnostics and Genomics Segment Customers and Distribution Methods
The customers for the Spatial Biology division include researchers in academia as well as investigators in pharmaceutical and biotech companies. We sell our products directly to those customers who are primarily located in North America, Europe and China, and through distributors elsewhere. In addition to being useful research tools, our DNA and RNA in situ hybridization (ISH) assays have diagnostics applications as well, and several are cleared or currently under review by the FDA in partnership with diagnostics instrument manufacturers and pharmaceutical companies.
In the United States, we offer the ExosomeDx Prostate test to physicians using our lab-developed non-invasive urine-based assay for prostate cancer detection. Our diagnostic laboratory is certified under and regulated by the State of Massachusetts pursuant to the Clinical Laboratory Improvement Amendments, or CLIA. We reach our customers through physicians prescribing such tests for their patients. This test is also available in Europe as a CE-marked product. The Asuragen-branded products are sold primarily to laboratories for use in lab-developed tests or in kit form as regulated diagnostic tests.
The majority of Diagnostic Reagents Division’s sales are through OEM agreements, but we sell some of our diagnostic reagent products directly to customers and, in Europe and Asia, also through distributors.
No customer accounted for 10% or more of the reporting segment’s consolidated net sales during fiscal years 2022, 2021 or 2020.
MANUFACTURING AND MATERIALS
Our manufacturing operations use a wide variety of raw materials and components, including electronic components, chemicals and biological materials. No single supplier is material, although for some components that require particular
8
specifications or regulatory or other qualifications there may be a single supplier or a limited number of suppliers that can readily provide such components. We utilize a number of techniques to address potential disruption in and other risks relating to our supply chain, which in certain cases includes, the use of safety stock, alternative materials, and qualification of multiple supply sources.
The majority of our products are shipped within one day of receipt of the customers’ orders, other than our instruments and related cartridges, which are typically shipped within one to two weeks of receipt of an order. There was no significant backlog of orders for our products as of the date of this Annual Report on Form 10-K or as of a comparable date for fiscal 2022. For additional discussion of risks relating to supply chain and manufacturing, refer to “Item 1A. Risk Factors.”
COMPETITION
Although our segments both generally operate in highly competitive markets, it is difficult to determine our competitive position, either in the aggregate or by segment, since none of our competitors offer all of the same product and service lines or serve all of the same markets as the Company, or any of its segments, does. Because of the range of the products and services we sell, we encounter a wide variety of competitors, including a number of large, global companies or divisions of such companies with substantial capabilities and resources, as well a number of smaller, niche competitors with specialized product offerings. We have seen increased competition in a number of our markets as a result of the entry of new companies into certain markets, the entry of competitors based in low-cost manufacturing locations, and increasing consolidation in particular markets. The number of competitors varies by product line. Key competitive factors vary among the Company’s businesses, but include the specific factors noted above with respect to each particular business and typically also include price, quality and safety, performance, delivery speed, application expertise, service and support, technology and innovation, distribution network, breadth of product, service and software offerings, and brand name recognition. We believe our competitive position is strong due to the unique aspects of many of our products and our product quality. For a discussion of risks related to competition, refer to “Item 1A. Risk Factors.”
SEASONALITY OF BUSINESS
Bio-Techne believes there is some seasonality as a result of vacation and academic schedules of its worldwide customer base, particularly for the Protein Sciences segment.
There is also some seasonality for the ExosomeDx Prostate test, as patients tend to avoid scheduling medical appointments during the summer and other holidays. A majority of Diagnostics Reagents division products are manufactured in large bulk lots and sold on a schedule set by the customer. Consequently, sales for that division can be unpredictable, and not necessarily based on seasonality. As a result, we can experience material and sometimes unpredictable fluctuations in our revenue from the Diagnostics and Genomics segment.
GOVERNMENT CONTRACTS
Although the Company transacts business with various government entities, no government contract is of such magnitude that renegotiation of profits or termination of the contract at the election of the government entity would have a material adverse effect on the Company’s financial results. As a party to these contracts, Bio-Techne does have to comply with certain regulations that apply to companies doing business with governments. For a discussion of risks related to government contracting requirements, see “Item 1A. Risk Factors.”
NEW PRODUCTS AND RESEARCH AND DEVELOPMENT
We believe that our future success depends, to a large extent, on our ability to keep pace with changing technologies and market needs. Bio-Techne is engaged in continuous research and development in all of our major product lines. We also carry out research to develop new products that build upon and expand the technologies we acquire through our acquisition strategy. In fiscal 2022, we introduced over 1,000 new products. While this is an area of focus for the Company, there is no assurance that any of the products in the research and development phases can be successfully completed or, if completed, can be successfully introduced into the marketplace.
9
HUMAN CAPITAL
Through its subsidiaries, Bio-Techne employed approximately 3,000 full-time and part-time employees as of June 30, 2022, of whom approximately 2,300 were employed in the United States and approximately 650 outside the United States. None of the United States employees are unionized. Outside the United States, the Company has government-mandated collective bargaining arrangements or work councils in certain countries.
Bio-Techne is committed to attracting, developing, engaging and retaining the best people possible from around the world to sustain and grow our leadership position in life sciences tools and diagnostics. We strive to create an employee experience that allows each to achieve their life’s best work. This is demonstrated by leading with our EPIC values of Empowerment, Passion, Innovation and Collaboration. We continuously build on our people-first culture, led by uncompromising integrity, hosting a place of belonging, granting access to innovation and respecting human rights around the globe.
Our talent management strategy spans multiple key dimensions, including the following:
Culture and Governance
Our four EPIC values of Empowerment, Passion, Innovation and Collaboration are the backbone for the way we approach the leadership and direction of our work force. Employees are empowered to realize their potential. Our culture supports and encourages a collaborative approach to working with each other and with our customers. We encourage innovation to continually improve our products, services and processes, and our passions for science and the missions of our customers are our guiding lights.
Our EPIC values are embedded in our culture and practices. For example, our performance management system and annual review processes incorporate our EPIC values. Each employee is measured against the behaviors and attributes that support those values. To further amplify our desired behaviors, we have an annual employee recognition program in which we ask for nominations and recognize winning individuals and teams form across our business who have best demonstrated our EPIC values.
Bio-Techne’s Board of Directors reviews management succession planning at least annually, and its Compensation Committee reviews the Company’s talent management strategy periodically in connection with significant initiatives and acquisitions, as well as part of its oversight of our executive and equity compensation programs. At the management level, our Chief Human Resources Officer, who reports directly to our President and CEO, is responsible for the development and execution of the Company’s talent management strategy.
Engagement and Belonging
Our engagement strategy focuses on developing the best workplace and best people leaders to meet our employees’ needs. We believe that strong employee engagement helps enable higher retention and better business performance. We also engage more formally via an annual engagement survey that assesses our employees’ overall experience. In 2021, 73% of our global workforce participated, and 87% of those who responded provided positive feedback. While these responses were positive, our management used the responses to inform and shape our future employee-focused initiatives. These initiatives in the past have resulted in changes in programs and policies, including expansion of our management and leadership development programs, addition of a parental leave program, expansion of our incentive programs to include annual cash bonuses to all employees, introduction of flexible working and expanding the breadth of our Employee Resource Groups (ERGs).
We believe a diverse workforce and culture of belonging are both essential to drive innovation, fuel growth and help ensure our technologies and products effectively serve a global customer base. The Company’s executive-sponsored Belonging initiative is focused on providing a welcoming working environment for all employees, continued education, broadening our candidate pools, and implementing and sustaining programs. One of the centerpieces of our talent development strategy is our ERGs. They offer mentorship, support and engagement to help our employees, including those from underrepresented groups, succeed and thrive. As of June 30, 2022, we had 10 ERGs operating globally.
10
As of June 30, 2022, 49% of our total employee population was female, and 46% of our managerial employees were female. In the United States, 37% of our total employee population identified as nonwhite and 36% of our managerial employees identified as nonwhite.
Recruitment and Retention
We believe that sustaining our profitable growth will require a continued focus on recruiting and retaining top, diverse talent. We engage in a variety of recruiting strategies intended to locate and identify qualified candidates, and to maintain a talent pipeline. The Company offers competitive pay and benefits, from flexible work to financial planning resources to an employee stock purchase plan. In fiscal 2022, we bolstered our recruitment and retention efforts by expanding eligibility to receive stock options and annual cash bonuses.
In addition to pay and benefits, we believe one key to retention is to maintain an environment in which employees can work productively and enjoy opportunities to develop and advance. The Company seeks to cultivate a culture of empowerment and collaboration, allowing employees to understand the impact of their efforts and see opportunities for career growth. We believe that our focus and investment in recruitment and retention contributed to our inclusion on the Forbes list as one of America’s Best Midsize Employers as well as one of the Best Employers for Diversity.
The last fiscal year saw considerable employee turnover in all industries, including the biotechnology industry, and we were able to adapt and respond to turnover pressures in our industry to deliver strong growth and profitability. We believe our sustained efforts to enhance recruitment and retention will allow us to remain resilient and productive in the face of increased employee mobility and economic challenge.
Talent Development and Learning and Development
Bio-Techne invests in people development with the belief that growing and promoting employees from within the Company creates a more sustainable organization. High potential and promotable employees are identified through our annual talent review strategy. These employees are elevated to the attention of senior management and may be considered for additional development and career advancement opportunities.
Our global learning and development program delivers a wide range of initiatives including a validated suite of compliance training, and soft, technical, business, interpersonal and career skills. Many of these programs are assigned to individuals specifically. In addition, there are some programs available to employees in order to accelerate their own development. As a company that regularly acquires other businesses, we believe it is important for employees to be trained in the skills and mindsets that enable them to respond positively to change. This initiative allows individuals to deal with change easily and reduces the need to run large scale change management programs.
Well-Being and Safety
The Company is committed to protecting the physical health and psychological well-being of our employees by providing a safe work environment. We train all employees on foundational safety principles and require more rigorous safety and hazard awareness training where appropriate based on function, role, or team. All employees are empowered and encouraged to maintain and create a safe workplace. In addition, we offer internal and external resources to provide for the psychological and emotional security of employees, including employee resource programs and mental health benefit coverage.
The COVID-19 pandemic imposed new and unusual challenges in maintaining a safe workplace. As an essential business providing key research and diagnostics products needed to confront the pandemic, Bio-Techne maintained operations while providing a safe work environment through staggered shifts, work from home protocols where possible, masking and vaccine requirements, and other significant safety measures.
Community
The Company believes in giving back and in supporting the local communities in which we live and work. The Company and its employees donate financially and by giving their time and energy. Most sites or departments engage in local charitable causes and activities. In some of our sites, employees are encouraged to give through regular payroll deductions
11
and through the annual campaign week where employee contributions are matched by the Company. Some charitable causes are identified and promoted by our ERGs. In addition, United States employees receive eight hours of voluntary paid time off to participate in local opportunities to give back to the community.
INTELLECTUAL PROPERTY
Our success depends in part upon our ability to protect our core technologies and intellectual property. To accomplish this, we rely on a combination of intellectual property rights, including patents, trade secrets and trademarks, as well as customary contractual protections in our terms and conditions and other sales-related documentation.
As of June 30, 2022, we had rights to approximately 440 granted patents and approximately 270 pending patent applications. Products in the Analytical Solutions and Genomics divisions are protected primarily through pending patent applications and issued patents. In addition, certain of our products are covered by licenses from third parties to supplement our own patent portfolio. Patent protection, if granted, generally has a life of 20 years from the date of the patent application or patent grant. We cannot provide assurance that any of our pending patent applications will result in the grant of a patent, whether the examination process will require us to narrow our claims, and whether our claims will provide adequate coverage of our competitors’ products or services.
In addition to pursuing patents on our products, we also preserve much of our innovation as trade secrets, particularly in the Reagent Solutions division of our Protein Sciences segment. Where appropriate, we use trademarks or registered trademarks in connection with our products. We have taken steps to protect our intellectual property and proprietary technology, in part by entering into confidentiality agreements and intellectual property assignment agreements with our employees, consultants, corporate partners and, when needed, our advisors. See the description of risks associated with the Company’s intellectual property in “Item 1A. Risk Factors.”
We can give no assurance that Bio-Techne’s products do not infringe upon patents or proprietary rights owned or claimed by others. Bio-Techne has not conducted a patent infringement study for each of its products. Where we have been contacted by patent holders with certain intellectual property rights, Bio-Techne typically has entered into licensing agreements with patent holders under which it has the exclusive and/or non-exclusive right to use patented technology as well as the right to manufacture and sell certain patented products to the research and/or diagnostics markets.
All trademarks, trade names, product names, graphics and logos of Bio-Techne contained herein are trademarks and registered trademarks of Bio-Techne or its subsidiaries, as applicable, in the United States and/or other countries. Solely for convenience, we may refer to trademarks in this Annual Report on Form 10-K without the ™ or ® symbols. Such references are not intended to indicate that we will not assert our full rights to our trademarks.
LAWS AND REGULATIONS
Our operations, and some of the products we offer, are subject to a number of complex laws and regulations governing the production, marketing, handling, transportation, and distribution of our products and services. The following sections describe certain significant regulations pertinent to the Company. These are not the only laws and regulations applicable to the Company’s business. For a description of risks related to laws and regulations to which we are subject, refer to “Item 1A. Risk Factors.”
Medical Device Regulations
A number of our products are classified as medical devices and are subject to restrictions under domestic and foreign laws, rules, regulations, self-regulatory codes and orders, including but not limited to the U.S. Food, Drug and Cosmetic Act (the “FDCA”). The FDCA requires these products, when sold in the United States, to be safe and effective for their intended uses and to comply with the regulations administered by the U.S. Food and Drug Administration (“FDA”). The FDA regulates the design, development, testing, manufacture, advertising, labeling, packaging, marketing, distribution, import and export and record keeping for such products. Many medical device products are also regulated by comparable agencies in non-U.S. countries in which they are produced or sold.
Any medical devices we manufacture and distribute are subject to pervasive and continuing regulation by the FDA and certain state and non-U.S. agencies. As a medical device manufacturer, our manufacturing facilities are subject to
12
inspection on a routine basis by the FDA. We are required to adhere to the Current Good Manufacturing Practices (“cGMP”) requirements, as set forth in the Quality Systems Regulation (“QSR”), which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all phases of the design and manufacturing process.
We must also comply with post-market surveillance regulations, including medical device reporting (“MDR”), requirements which require that we review and report to the FDA any incident in which our products may have caused or contributed to a death or serious injury. We must also report any incident in which our product has malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur.
Labeling and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Medical devices approved or cleared by the FDA may not be promoted for unapproved or uncleared uses, otherwise known as “off-label” promotion. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses.
In the European Union (“EU”), our products are subject to the medical device laws of the various member states, which are currently based on a Directive of the European Commission. Additionally, the EU has adopted the In Vitro Diagnostic Regulation (the “EU IVDR”), which imposes stricter requirements for the marketing and sale of in vitro diagnostic medical devices, including in the area of clinical evaluation requirements, quality systems and post-market surveillance. Manufacturers of in vitro diagnostics medical devices that have been marketed and sold under the prior regulatory regime now have to comply with some of the new EU IVDR requirements, while the effective date of other requirements have been delayed. Complying with EU IVDR may require material modifications to our quality management systems, additional resources in certain functions, updates to technical files and additional clinical data in some cases, among other changes.
One of our products under our Exosome Diagnostics brand is offered as a test by a certified laboratory under CLIA. Our Asuragen business also maintains a CLIA certification. Consequently, we must comply with state licensing regulations applicable to laboratories regulated under CLIA, governing laboratory practices and procedures.
Other Healthcare Laws
Several of the products sold in our Diagnostics and Genomics segment are subject to various health care related laws regulating fraud and abuse, research and development, pricing and sales and marketing practices, and the privacy and security of health information, including, among others:
| ● | U.S. federal regulations regarding quality and cost by the U.S. Department of Health and Human Services (“HHS”), including the Centers for Medicare & Medicaid Services (“CMS”), as well as comparable state and non-U.S. agencies responsible for reimbursement and regulation of healthcare goods and services, including laws and regulations related to kickbacks, false claims, self-referrals and healthcare fraud. |
13
For a discussion of risks related to regulation by the FDA and comparable agencies of other countries, and the other regulatory regimes referenced above, please refer to section entitled “Item 1A. Risk Factors.”
Data Privacy and Security Laws
As a global organization, we are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal and/or sensitive data in the course of our business. In addition to the U.S. HIPAA privacy and security rules mentioned above, which impact some parts of our business, individual states also regulate data breach and security requirements, and multiple governmental bodies assert authority over aspects of the protection of personal privacy. In particular, a broad privacy law in California, the California Consumer Privacy Act (“CCPA”), came into effect in January 2020. The CCPA has some of the same features as the GDPR (discussed below) and has already prompted several other states to follow with similar laws. The EU General Data Protection Regulation that became effective in May 2018 (“GDPR”) has imposed significantly stricter requirements in how we collect, transmit, process, and retain personal data, including, among other things, in certain circumstances a requirement for almost immediate notice of data breaches to supervisory authorities and prompt notice to data subjects with significant fines for non-compliance. Several other countries in which we do business have passed, and other countries are considering passing, laws that require personal data relating to their citizens to be maintained on local servers and impose additional data transfer restrictions. For a discussion of risks related to improper disclosure of private information particularly as a result of cyber security incidents, please refer to section entitled “Item 1A. Risk Factors.”
Environmental Health and Safety Laws
We are also subject to various environmental health and safety laws and regulations both within and outside the U.S. Like other companies in our industry, our manufacturing and research activities involve the use and transportation of substances regulated under environmental health and safety laws including those relating to the transportation of hazardous materials.
Other Laws and Regulations Governing Our Sales, Marketing and Shipping Activities
We are subject to the U.S. Foreign Corrupt Practices Act and various other similar anti-corruption and anti-bribery acts, which are particularly relevant to our operations in countries where the customers are government entities or are controlled by government officials. Both directly and indirectly through our distributors, we must comply with such laws when interacting with those entities.
As Bio-Techne’s businesses also include export and import activities, we are subject to pertinent laws enforced by the U.S. Departments of Commerce, State and Treasury. Other nations’ governments have implemented similar export/import control and economic sanction regulations, which may affect the Company’s operations or transactions subject to their jurisdictions.
In addition, under U.S. laws and regulations, U.S. companies and their subsidiaries and affiliates outside the United States are prohibited from participating or agreeing to participate in unsanctioned foreign boycotts in connection with certain business activities, including the sale, purchase, transfer, shipping or financing of goods or services within the United States or between the United States and countries outside of the United States. If we, or certain third parties through which
14
we sell or provide goods or services, violate anti-boycott laws and regulations, we may be subject to civil or criminal enforcement action and varying degrees of liability.
We are subject to laws and regulations governing government contracts, and failure to address these laws and regulations or comply with government contracts could harm our business by a reduction in revenue associated with these customers. We have agreements relating to the sale of our products to government entities and, as a result, we are subject to various statutes and regulations that apply to companies doing business with the government. We are also subject to investigation for compliance with the regulations governing government contracts. A failure to comply with these regulations could result in suspension of these contracts, criminal, civil and administrative penalties or debarment.
For a discussion of risks related to the above-referenced regulations, particularly with respect to our international operations, please refer to section entitled “Item 1A. Risk Factors.”
INVESTOR INFORMATION
We are subject to the information requirements of the Securities Exchange Act of 1934 (the Exchange Act). Therefore, we file periodic reports, proxy statements, and other information with the Securities and Exchange Commission (SEC). The SEC maintains an internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically.
Financial and other information about us is available on our web site (https://investors.bio-techne.com/). We make available on our web site copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13 or 15(d) of the Exchange Act as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the SEC.
EXECUTIVE OFFICERS OF THE REGISTRANT
Currently, the names, ages, positions and periods of service of each executive officer of the Company are as follows:
Name |
| Age |
| Position |
| Officer Since |
Charles Kummeth |
| 62 |
| President, Chief Executive Officer and Director |
| 2013 |
James Hippel |
| 51 |
| Executive Vice President and Chief Financial Officer |
| 2014 |
Brenda Furlow |
| 64 |
| Executive Vice President, General Counsel and Corporate Secretary |
| 2014 |
Kim Kelderman |
| 55 |
| President, Diagnostics and Genomics |
| 2018 |
William Geist |
| 52 |
| President, Protein Sciences |
| 2022 |
Set forth below is information regarding the business experience of each executive officer. There are no family relationships among any of the officers named, nor is there any arrangement or understanding pursuant to which any person was selected as an officer.
Charles Kummeth has been President and Chief Executive Officer of the Company since April 1, 2013. Prior to joining the Company, he served as an executive at Thermo Fisher Scientific and in various roles at 3M Corporation.
James Hippel has been Chief Financial Officer of the Company since April 1, 2014. Prior to joining the Company, Mr. Hippel served as Senior Vice President and Chief Financial Officer for Mirion Technologies, Inc and as Vice President, Finance at Thermo Fisher Scientific, and in financial roles at Honeywell International. Mr. Hippel started his career with KPMG LLP.
Brenda Furlow joined the Company as General Counsel and Corporate Secretary on August 4, 2014. Prior to joining Bio-Techne, Ms. Furlow served as general counsel for TomoTherapy, Inc. and Promega Corporation.
Kim Kelderman joined Bio-Techne on April 30, 2018 as President, Diagnostics and Genomics. Prior to Bio-Techne, Mr. Kelderman was an executive at Thermo Fisher Scientific and a Senior Segment Leader at Becton Dickinson.
William Geist has been President of the Protein Sciences segment since January 3, 2022. Prior to Bio-Techne, Mr. Geist most recently served as Chief Operating Officer for Quanterix, and before that in senior management roles at Thermo Fisher Scientific and QuantaBiosciences, a QIAGEN company.
15
ITEM 1A. RISK FACTORS
Set forth below are risks and uncertainties we believe are material to our investors. You should refer to the explanation of the qualifications and limitations on forward-looking statements in the section titled Information Relating to Forward-Looking Statements at the beginning of this Annual Report on Form 10-K.
Economic and Industry Risks
Conditions in the global economy, the particular markets we serve and the financial markets, whether brought about by material global crises or other factors, may adversely affect our business and financial results.
Our business is sensitive to global economic conditions. Slower economic growth in the domestic or international markets, inflation, recession, volatility in the credit and currency markets, high levels of unemployment or underemployment, labor availability constraints, changes or anticipation of potential changes in government trade, fiscal, tax or monetary policies, government budget dynamics (particularly in the healthcare and scientific research areas), and other challenges in the global economy have in the past adversely affected, and may in the future adversely affect, the Company and its distributors, customers, and suppliers. In the past three years, COVID-19 has had, and likely will continue to have, an adverse impact on the global economy, including as a result of impacts associated with protective health measures that we, other businesses and governments are taking or might have to take again in the future to manage the pandemic For example, as the world has grappled with the COVID-19 pandemic, some governments, including the People’s Republic of China, have continued to impose strict “stay-at-home” orders to manage the pandemic, which have significantly impacted the economy in that country and our business there. Should these restrictions continue in China or if they are imposed again elsewhere, our business could be materially impacted.
Without limiting the foregoing, we have experienced and/or may in the future experience:
If growth in the global economy or in any of the markets we serve slows for a significant period, if there is significant deterioration in the global economy or such markets or if improvements in the global economy do not benefit the markets we serve, our business and financial results can be adversely affected.
International political, compliance and business factors, including the military conflict in Ukraine and the United Kingdom’s withdrawal from the European Union, can negatively impact our operations and financial results.
We engage in business globally, with approximately 42% of our sales revenue in fiscal 2022 coming from outside the U.S. Changes, potential changes or uncertainties in social, political, regulatory and economic conditions or laws and policies
16
governing foreign trade, manufacturing, and development and investment in the territories and countries where we or our customers operate, or governing the health care system, can adversely affect our business and financial results. For example, Congress and the U.S. administration are also considering significant changes to healthcare in the United States, including government negotiation/regulation of drug prices paid by government programs. Such impacts could negatively impact certain markets we serve, resulting in adverse impact on our sales revenue.
Political and military conflicts may disrupt our business or negatively impact global economic or business conditions. For example, Russia’s military invasion of Ukraine, and the response by the US and European countries to that invasion, have caused severe political, humanitarian and economic crises, not only in Europe but globally. Restrictions on trade, particularly involving certain foods and energy supplies, have increased prices, led to widespread inflation and otherwise aggravated the economic challenges resulting from the COVID-19 pandemic. While we have not historically had significant business in either Russia or Ukraine, the broader impact of the conflict could negatively impact our operations and financial results.
Additionally, the UK’s exit from the European Union at the end of calendar year 2020 continues to create political and economic uncertainty, particularly in the UK and the EU, having disrupted the free flow of goods and people between the UK and the EU. In addition, our business could be negatively affected by new trade agreements between the UK and other countries, including the United States, and by the possible imposition of trade or other regulatory barriers in the UK. Any of these factors have affected and could continue to adversely affect customer demand, our relationships with customers and suppliers, and our business and financial results, particularly since our European headquarters and primary shipping facilities have traditionally been centered in the UK.
One of our strategies is to expand geographically, particularly in China, India and in developing countries, both through distribution and through direct operations. This subjects us to a number of risks, including international economic, political, and labor conditions; currency fluctuations; tax laws (including U.S. taxes on foreign subsidiaries); increased financial accounting and reporting burdens and complexities; unexpected changes in, or impositions of, legislative or regulatory requirements; failure of laws to protect intellectual property rights adequately; inadequate local infrastructure and difficulties in managing and staffing international operations; delays resulting from difficulty in obtaining export licenses for certain technology; tariffs, quotas and other trade barriers and restrictions; transportation delays; operating in locations with a higher incidence of corruption and fraudulent business practices; and other factors beyond our control, including terrorism, war, natural disasters, climate change and diseases.
The application of laws and regulations impacting global transactions is often unclear and may at times conflict. Compliance with these laws and regulations may involve significant costs or require changes in our business practices that result in reduced revenue and profitability. Non-compliance could also result in fines, damages, criminal sanctions, prohibited business conduct, and damage to our reputation. We incur additional legal compliance costs associated with our global operations and could become subject to legal penalties in foreign countries if we do not comply with local laws and regulations, which may be substantially different from those in the U.S.
We continue to expand our operations in countries with developing economies, where it may be common to engage in business practices that are prohibited by U.S. regulations applicable to the Company, such as the Foreign Corrupt Practices Act. Although we implement policies and procedures designed to ensure compliance with these laws, there can be no assurance that all of our employees, contractors, and agents, as well as those companies to which we outsource certain aspects of our business operations, including those based in foreign countries where practices which violate such U.S. laws may be customary, will comply with our internal policies. Any such non-compliance, even if prohibited by our internal policies, could have an adverse effect on our business and result in significant fines or penalties.
The healthcare and life sciences industries that we serve face constant pressures and changes in an effort to reduce healthcare costs or increase their predictability, all of which may adversely affect our business and financial results.
Our Protein Sciences segment products are sold primarily to research scientists at pharmaceutical and biotechnology companies and at university and government research institutions. In addition to the impacts described above relating to COVID-19, research and development spending by our customers and the availability of government research funding can fluctuate due to changes in available resources, mergers of pharmaceutical and biotechnology companies, spending priorities, general economic conditions and institutional and governmental budgetary policies. We carry essentially no
17
backlog of orders and changes in the level of orders received and filled daily can cause fluctuations in quarterly revenues and earnings.
Our Genomics and Diagnostics segment includes products for the medical diagnostics market, which relies largely on government healthcare-related policies and funding. Changes in government reimbursement for certain diagnostic tests or reductions in overall healthcare spending could negatively impact us directly or our customers and, correspondingly, our sales to them. For example, our Exosome Diagnostics business develops and sells novel exosome-based diagnostic tests. While we received public payer coverage for certain uses, we are currently seeking expanded coverage from public payors as well as coverage decisions regarding reimbursement from additional private payers. However, the process and timeline for obtaining coverage decisions is uncertain and difficult to predict. Further, reimbursement reductions due to changes in policy regarding coverage of tests or other requirements for payment (such as prior authorization, diagnosis code and other claims edits, or a physician or qualified practitioner’s signature on test requisitions) may be implemented from time to time. All of these payor actions and changes may have a material adverse effect on revenue and earnings associated with our diagnostics products.
Acquisition and Investment Risks
Our inability to complete acquisitions at our historical rate and at appropriate prices, and to make appropriate investments that support our long-term strategy, could negatively impact our growth rate and stock price.
One of our key strategies is growth through acquisition of other businesses and assets. Our ability to grow revenues, earnings and cash flow at or above our historic rates depends in part upon our ability to identify and successfully acquire and integrate businesses at appropriate prices and realize anticipated synergies, and to make appropriate investments that support our long-term strategy. We may not be able to consummate acquisitions at rates similar to the past, which could adversely impact our growth rate and our stock price. Promising acquisitions and investments are difficult to identify and complete for a number of reasons, including high valuations, competition among prospective buyers or investors, the availability of affordable funding in the capital markets and the need to satisfy applicable closing conditions and obtain applicable antitrust and other regulatory approvals on acceptable terms. Changes in accounting or regulatory requirements or instability in the credit markets could also adversely impact our ability to consummate acquisitions and investments.
Our acquisition of businesses, investments, joint ventures and other strategic relationships, if not properly implemented or integrated, could negatively impact our business and financial results.
As part of our business strategy we acquire businesses, make investments and enter into joint ventures and other strategic relationships in the ordinary course, and we also from time to time complete more significant transactions. We joined with two partners to establish a collaborative marketing venture, ScaleReady LLC, to address the needs of the rapidly expanding cell and gene therapy market, and subsequently announced that we had entered into an option agreement to potentially invest in and then acquire one of those partners, Wilson Wolf Manufacturing. More recently, subsequent to the end of our fiscal year, we acquired Namocell Inc., a single cell sorting and dispensing platform company While we believe these business ventures will advance our business strategies and support our growth plans, we may not be successful in managing or integrating them into our company. Acquisitions, investments, joint ventures and strategic relationships involve a number of additional financial, accounting, managerial, operational, legal, compliance and other risks and challenges, including but not limited to the following, any of which could adversely affect our business and our financial results:
18
| ● | we may be unable to achieve cost savings or other synergies anticipated in connection with an acquisition, investment, joint venture or strategic relationship; |
| ● | we have assumed and may assume unknown liabilities, known contingent liabilities that become realized, known liabilities that prove greater than anticipated, internal control deficiencies or exposure to regulatory sanctions resulting from the acquired company’s or investee’s activities and the realization of any of these liabilities or deficiencies can increase our expenses, adversely affect our financial position or cause us to fail to meet our public financial reporting obligations; |
| ● | in connection with acquisitions and joint ventures, we often enter into post-closing financial arrangements such as purchase price adjustments, earn-out obligations and indemnification obligations, which can have unpredictable financial results; and |
| ● | investing in or making loans to early-stage companies often entails a high degree of risk, and we may not always achieve the strategic, technological, financial or commercial benefits we anticipate; we may lose our investment or fail to recoup our loan; or our investment may be illiquid for a greater-than-expected period of time. |
We may be required to record a significant charge to earnings if our goodwill and other amortizable intangible assets or other investments become impaired, which could negatively impact our financial results or stock price.
We are required under generally accepted accounting principles to test goodwill for impairment at least annually and to review our goodwill, amortizable intangible assets, and other assets acquired through merger and acquisition activity for impairment when events or changes in circumstance indicate the carrying value may not be recoverable. Factors that could lead to impairment of goodwill, amortizable intangible assets, and other assets acquired via acquisitions include significant adverse changes in the business climate and actual or projected operating results (affecting our company as a whole or affecting any particular segment) and declines in the financial condition of our business. We may be required in the future to record additional charges to earnings if our goodwill, amortizable intangible assets or other investments become impaired. Any such charge would adversely impact our financial results.
In addition, the Company’s expansion strategies include collaborations and investments in joint ventures and companies developing new products related to the Company’s business. These strategies carry risks that objectives will not be achieved and future earnings will be adversely affected.
Strategic and Operational Risks
Our success will be dependent on recruiting and retaining highly qualified and diverse personnel and creating and maintaining a culture that successfully integrates the employees joining through acquisitions.
Recruiting and retaining qualified scientific, production, sales and marketing, and management personnel representing diverse backgrounds, experiences and skill sets are critical to our success. The market for highly skilled workers and leaders in our businesses, particularly in the areas of science and technology, is extremely competitive. In fiscal 2022, a number of our businesses and departments faced labor availability constraints and inflationary costs. In general, we have been experiencing turnover at higher rates than usual and have had some difficulties filling certain positions. In particular, we operate in several geographic locations where competition for talent is strong, making employee retention even more challenging. For example, some of our fastest growing businesses are located in California and Massachusetts, both of which in the last several years have had low unemployment and a particularly competitive environment for finding and retaining talent. Our growth by acquisition also creates challenges in retaining employees. As we integrate past and future acquisitions and evolve our corporate culture to incorporate the new workforces, some employees may not find such integration or cultural changes appealing. Finally, as the geographies in which we operate recover from the recent
19
pandemic and we return employees who had been working from home back to our sites, we may not be able to retain people who prefer continuing to work from home full time. The failure to attract and retain such personnel could adversely affect our business.
Our growth depends in part on the timely development and commercialization of new and enhanced products and services that meet our customers’ needs. Our growth can also be negatively impacted if our customers do not grow as anticipated.
We generally sell our products and services in industries that are characterized by rapid technological change, frequent new product introductions and new market entrants and competitors. If we do not develop innovative new and enhanced products and services on a timely basis, our offerings will become obsolete over time and our business and financial results will suffer. Our success will depend on several factors, including our ability to:
| ● | obtain necessary regulatory approvals of appropriate scope (including with respect to certain diagnostic medical device products by demonstrating satisfactory clinical results where applicable, as well as achieving third-party reimbursement); and |
If we fail to accurately predict future customer needs and preferences or fail to produce viable technologies, we may invest heavily in research and development of products that do not lead to significant revenue, which would adversely affect our business and financial results. Even when we successfully innovate and develop new and enhanced products, we often incur substantial costs in doing so, and our profitability may suffer.
We face intense competition, and if we are unable to compete effectively, we may experience decreased demand and decreased market share or need to reduce prices to remain competitive.
We face intense competition across most of our product lines. Competitors include companies ranging from start-up companies, which may be able to more quickly respond to customers’ needs, to large multinational companies, which may have greater financial, marketing, operational, and research and development resources than us. In addition, consolidation trends in the pharmaceutical, biotechnology and diagnostics industries have served to create fewer customer accounts and to concentrate purchasing decisions for some customers, resulting in increased pricing pressure on us. Moreover, customers may believe that consolidated businesses are better able to compete as sole source vendors, and therefore prefer to purchase from such businesses. The entry into the market by manufacturers in countries in Asia and other low-cost manufacturing locations is also creating increased pricing and competitive pressures, particularly in developing markets. In order to compete effectively, we must retain longstanding relationships with major customers and continue to grow our business by establishing relationships with new customers, continually developing new products and services to maintain and expand our brand recognition and leadership position in various product and service categories and penetrating new
20
markets, including high-growth markets. Our ability to compete can also be impacted by changing customer preferences and requirements (for example increased demand for more environmentally-friendly products and supplier practices). Our failure to compete effectively and/or pricing pressures resulting from competition may adversely impact our business and financial results, and our expansion into new markets may result in greater-than-expected risks, liabilities and expenses.
A significant disruption in, or breach of security of, our information technology systems or data, or violation of data privacy laws, could result in damage to our reputation, data integrity and/or subject us to costs, fines, or lawsuits under data privacy or other laws or contractual requirements.
The integrity and protection of our own data, and that of our customers and employees, is critical to our business. We rely on information technology systems, some of which are provided and/or managed by third parties, to process, transmit and store electronic information (including sensitive data such as confidential business information and personally identifiable data relating to employees, customers, other business partners and patients), and to manage or support a variety of critical business processes and activities (such as receiving and fulfilling orders, billing, collecting and making payments, shipping products, providing services and support to customers and fulfilling contractual obligations). These systems, products and services (including those we acquire through business acquisitions) can be damaged, disrupted or shut down due to attacks by computer hackers, computer viruses, ransomware, human error or malfeasance, power outages, hardware failures, telecommunication or utility failures, catastrophes or other unforeseen events, and in any such circumstances our system redundancy and other disaster recovery planning may be ineffective or inadequate. Attacks can also target hardware, software and information installed, stored or transmitted in our products after such products have been purchased and incorporated into third-party products, facilities or infrastructure. Security breaches of systems provided or enabled by us, regardless of whether the breach is attributable to a vulnerability in our products or services, or security breaches of third party systems we rely on to process, store or transmit electronic information, can result in the misappropriation, destruction or unauthorized disclosure of confidential information or personal data belonging to us or to our employees, partners, customers, patients or suppliers. These attacks, breaches, misappropriations and other disruptions and damage can interrupt our operations or the operations of our customers and partners, delay production and shipments, result in theft of our and our customers’ intellectual property and trade secrets, result in disclosure of personally identifiable information, damage customer, patient, business partner and employee relationships and our reputation and result in defective products or services, legal claims and proceedings, liability and penalties under privacy laws and increased costs for security and remediation, in each case resulting in an adverse effect on our business and financial results.
In addition, our information technology systems require an ongoing commitment of significant resources to maintain and enhance existing systems and develop or integrate new systems to keep pace with continuing changes in information processing technology, evolving legal and regulatory standards, evolving customer expectations, changes in the techniques used to obtain unauthorized access to data and information systems, and the information technology needs associated with our changing products and services. There can be no assurance that we will be able to successfully maintain, enhance and upgrade our systems as necessary to effectively address these requirements.
If we are unable to maintain reliable information technology systems or appropriate controls with respect to global data privacy and security requirements and prevent data breaches, we may suffer regulatory consequences in addition to business consequences. As a global organization, we are subject to data privacy and security laws, regulations, and customer-imposed controls in numerous jurisdictions as a result of having access to and processing confidential, personal and/or sensitive data in the course of our business. For example, in the United States, a small number of our businesses are subject to HIPAA. Entities that violate HIPAA due to a breach of unsecured patient health information, or that arise from a complaint about privacy practices or an audit by the HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. Individual states regulate data breach and security requirements and multiple governmental bodies assert authority over aspects of the protection of personal privacy. Most notably, in the last several years, some states, including California, Virginia, Utah, Colorado and Connecticut, have passed broad privacy legislation that could result in more material impacts as new regulations are issued. European laws require us to have an approved legal mechanism to transfer personal data out of Europe. Failure to comply with the requirements of GDPR and the applicable national data protection laws of the EU member states may result in significant fines and other administrative penalties. Several other countries such as China and Russia have passed, and other countries are considering passing, laws that require personal data relating to their citizens to be maintained on local servers and impose additional data transfer restrictions. Government enforcement actions can be costly and interrupt the
21
regular operation of our business, and data breaches or violations of data privacy laws can result in fines, reputational damage and civil lawsuits, any of which may adversely affect our business, reputation and financial results.
If we suffer loss to our supply chains, distribution systems or information technology systems due to catastrophe or other events, our operations could be seriously harmed.
Our supply chains, distribution systems and information technology systems may be subject to catastrophic loss due to fire, flood, earthquake, hurricane, power shortage or outage, public health crisis (including epidemics and pandemics) and the reaction thereto, war, terrorism, riot or other natural or man-made disasters, such as the COVID-19 pandemic. If any of these supply chains or systems were to experience a catastrophic loss, it could disrupt our operations, delay production and shipments, result in defective products or services, diminish demand, damage customer relationships and our reputation and result in legal exposure and significant repair or replacement expenses. The third-party insurance coverage that we maintain varies from time to time in both type and amount depending on cost, availability and our decisions regarding risk retention, and may be unavailable or insufficient to protect us against such losses.
The manufacture of many of our products is a complex process, and if we directly or indirectly encounter problems manufacturing products, our business and financial results could suffer.
The manufacture of many of our products is a complex process, due in part to strict regulatory requirements for some of our products. Problems can arise during manufacturing for a variety of reasons, including equipment malfunction, failure to follow specific protocols and procedures, problems with reliable sourcing of raw materials or components, natural disasters and environmental factors, and if not discovered before the product is released to market can result in recalls and product liability exposure. Because of the quality requirements of some of our customers as well as stringent regulations of the FDA and similar agencies regarding the manufacture of certain of our products, alternative manufacturing or sourcing is not always available on a timely basis to replace such production capacity. Any of these manufacturing problems could result in significant adverse impacts to our business and financial results.
If we cannot adjust our manufacturing capacity or the purchases required for our manufacturing activities to reflect changes in market conditions and customer demand, our business and financial results may suffer. In addition, our reliance upon sole or limited sources of supply for certain materials, components and services can cause production interruptions, delays and inefficiencies.
We purchase materials, components and equipment from third parties for use in many of our manufacturing operations. Our profitability could be adversely impacted if we are unable to adjust our purchases to reflect changes in customer demand and market fluctuations, including those caused by seasonality or cyclicality. During a market upturn, suppliers from time to time extend lead times, limit supplies or increase prices. If we cannot purchase sufficient products at competitive prices and quality and on a timely enough basis to meet increasing demand, we may not be able to satisfy market demand, product shipments may be delayed, our costs may increase, or we may breach our contractual commitments and incur liabilities. Conversely, in order to secure supplies for the production of products, we sometimes enter into noncancelable purchase commitments with vendors, which can impact our ability to adjust our inventory to reflect declining market demands. If demand for our products is less than we expect, we may experience additional excess and obsolete inventories and be forced to incur additional charges and our business and financial results may suffer.
In addition, some of our businesses purchase certain requirements from sole or limited source suppliers for reasons of quality assurance, regulatory requirements, cost effectiveness, availability or uniqueness of design. If these or other suppliers encounter financial, operating or other difficulties or if our relationship with them changes, we might not be able to quickly establish or qualify replacement sources of supply. The supply chains for our businesses can also be disrupted by supplier capacity constraints, bankruptcy or exiting of the business for other reasons, decreased availability of key raw materials or commodities and external events such as natural disasters, pandemic health issues, war, terrorist actions, governmental actions (such as trade protectionism) and legislative or regulatory changes. Any of these factors can result in production interruptions, delays, extended lead times and inefficiencies. Because we cannot always immediately adapt our production capacity and related cost structures to changing market conditions, at times our manufacturing capacity exceeds or falls short of our production requirements. Any or all of these problems can result in the loss of customers, provide an opportunity for competing products to gain market acceptance and otherwise adversely affect our business and financial results.
22
The Company relies heavily on internal manufacturing and related operations to produce, package and distribute its products which, if disrupted, could materially impair our business operations. Our business could be adversely affected by disruptions at our sites.
The Company’s internal quality control, packaging and distribution operations support the majority of the Company’s sales. Since certain Company products must comply with FDA regulations and because in all instances, the Company creates value for its customers through the development of high-quality products, any significant decline in quality or disruption of operations for any reason could adversely affect sales and customer relationships, and therefore adversely affect the business. While we have taken certain steps to manage these operational risks, the Company’s future sales growth and earnings may be adversely affected by perceived disruption risks or actual disruptions.
We rely upon our manufacturing operations to produce many of the products we sell and our warehouse facilities to store products, pending sale. Any significant disruption of those operations for any reason, such as strikes or other labor unrest, power interruptions, fire, hurricanes or other events beyond our control could adversely affect our sales and customer relationships and therefore adversely affect our business. We have significant operations in California, near major earthquake faults, which make us susceptible to earthquake risk. Although most of our raw materials are available from a number of potential suppliers, our operations also depend upon our ability to obtain raw materials at reasonable prices. If we are unable to obtain the materials we need at a reasonable price, we may not be able to produce certain of our products or we may not be able to produce certain of these products at a marketable price, which could have an adverse effect on our results of operations.
Climate change, or legal or regulatory measures to address climate change, may negatively affect us.
Climate change resulting from increased concentrations of carbon dioxide and other greenhouse gases in the atmosphere could present risks to our operations. For example, we have significant operations in California, where serious drought has made water less available and more costly and has increased the risk of wildfires. Changes in climate patterns leading to extreme heat waves or unusual cold weather at some of our locations can lead to increased energy usage and costs, or otherwise adversely impact our facilities and operations and disrupt our supply chains and distribution systems. Concern over climate change can also result in new or additional legal or regulatory requirements designed to reduce greenhouse gas emissions or mitigate the effects of climate change on the environment. Any such new or additional legal or regulatory requirements may increase the costs associated with, or disrupt, sourcing, manufacturing and distribution of our products, which may adversely affect our business and financial results. In addition, any failure to adequately address stakeholder expectations with respect to environmental, social and governance (“ESG”) matters may result in the loss of business, adverse reputational impacts, diluted market valuations and challenges in attracting and retaining customers and talented employees. In addition, our adoption of certain standards or mandated compliance to certain requirements could necessitate additional investments that could impact our profitability.
Defects and unanticipated use or inadequate disclosure with respect to our products, or allegations thereof, can adversely affect our business and financial results.
Certain of our products and services are sold for use in diagnostics. For those products and services in particular, manufacturing or design defects in, unanticipated use of, safety or quality issues (or the perception of such issues) with respect to, “off label” use of, or inadequate disclosure of risks relating to the use of products and services that we make or sell (including items that we source from third-parties) can lead to personal injury, death, and/or property damage and adversely affect our business and financial results. These events can lead to recalls or safety alerts, result in the removal of a product or service from the market and result in product liability or similar claims being brought against us. Recalls, removals and product liability and similar claims (regardless of their validity or ultimate outcome) result in significant costs, as well as negative publicity and damage to our reputation that could reduce demand for our products and services. Our business can also be affected by studies of the utilization, safety and efficacy of medical device products and components that are conducted by industry participants, government agencies and others. Any of the above can result in the discontinuation of marketing of such products in one or more countries and give rise to claims for damages from persons who believe they have been injured as a result of product issues, including claims by individuals or groups seeking to represent a class.
23
Because we rely heavily on third-party package-delivery services, a significant disruption in these services or significant increases in prices may disrupt our ability to ship products, increase our costs and lower our profitability.
Most of our reagent products need to be stored and shipped at certain cold temperatures. Consequently, we ship a significant portion of our products to our customers by express mail or air delivery through package delivery companies, such as FedEx in the U.S. and DHL in Europe. If one or more of these third-party package-delivery providers were to experience a major work stoppage, preventing our products from being delivered in a timely fashion or causing us to incur additional shipping costs we could not pass on to our customers, our costs could increase and our relationships with certain of our customers could be adversely affected. In addition, if one or more of these third-party package-delivery providers were to increase prices, and we were not able to find comparable alternatives or make adjustments in our delivery network, our profitability could be adversely affected.
Intellectual Property Risks
We are dependent on maintaining our intellectual property rights. If we are unable to adequately protect our intellectual property, or if third parties infringe our intellectual property rights, we may suffer competitive injury or expend significant resources enforcing our rights.
Many of the markets we serve are technology-driven, and as a result intellectual property rights play a significant role in product development and differentiation. We own numerous patents, trademarks, copyrights, trade secrets and other intellectual property and licenses to intellectual property owned by others, which in aggregate are important to our business. The intellectual property rights that we obtain, however, are not always sufficiently broad and do not always provide us a significant competitive advantage, and patents may not be issued for pending or future patent applications owned by or licensed to us. In addition, the steps that we and our licensors have taken to maintain and protect our intellectual property do not always prevent it from being challenged, invalidated, circumvented, designed around or becoming subject to compulsory licensing. In some circumstances, enforcement is not available to us because an infringer has a dominant intellectual property position or for other business reasons. We also rely on nondisclosure and noncompetition agreements with employees, consultants and other parties to protect, in part, trade secrets and other proprietary rights. There can be no assurance that these agreements adequately protect our trade secrets and other proprietary rights and will not be breached, that we will have adequate remedies for any breach, that others will not independently develop substantially equivalent proprietary information or that third parties will not otherwise gain access to our trade secrets or other proprietary rights.
These risks are particularly pronounced in countries in which we do business that do not have levels of protection of corporate proprietary information, intellectual property, technology and other assets comparable to the United States. We operate globally, with manufacturing operations in China and the UK, and approximately 42% of our revenue in fiscal 2022 was from outside the United States. The laws, regulations and enforcement mechanisms in other countries may in some cases be less protective of our intellectual property rights. Our failure to obtain or maintain intellectual property rights that convey competitive advantage, adequately protect our intellectual property or detect or prevent circumvention or unauthorized use of such property and the cost of enforcing our intellectual property rights can adversely impact our business and financial results.
We may be involved in disputes to determine the scope, coverage and validity of others’ proprietary rights, or to defend against third-party claims of intellectual property infringement, any of which could be time-intensive and costly and may adversely impact our business.
Our success depends in part on our ability to operate without infringing the proprietary rights of others, and to obtain licenses where necessary or appropriate. We have obtained and continue to negotiate licenses to produce a number of products claimed to be owned by others. Since we have not conducted a patent infringement study for each of our products, it is possible that some of our products may unintentionally infringe patents of third parties.
We have been and may in the future be sued by third parties alleging that we are infringing their intellectual property rights. These lawsuits are expensive, take significant time, and divert management’s focus from other business concerns. If we are found to be infringing the intellectual property of others, we could be required to cease certain activities, alter
24
our products or processes or pay licensing fees. This could cause unexpected costs and delays which may have a material adverse effect on us. If we are unable to obtain a required license on acceptable terms, or unable to design around any third party patent, we may be unable to sell some of our products and services, which could result in reduced revenue. In addition, if we do not prevail, a court may find damages or award other remedies in favor of the opposing party in any of these suits, which may adversely affect our earnings.
Financial and Tax Risks
We have entered into and drawn on a revolving credit facility, and we may incur additional debt in the future. The burden of this additional debt could adversely affect us, make us more vulnerable to adverse economic or industry conditions, and prevent us from funding our expansion strategy.
We currently have a Credit Agreement that provides for a revolving credit facility of $600 million, which can be increased by an additional $200 million subject to certain conditions, and a term loan of $250 million. Borrowings under the Credit Agreement bear interest at a variable rate. As of August 19, 2022, the Company had drawn $346 million under the Credit Agreement.
The terms of the Credit Agreement and the burden of the indebtedness incurred thereunder could have negative consequences for us, such as:
The Credit Agreement also contains negative covenants that limit our ability to engage in specified types of transactions. These covenants limit our ability to, among other things, sell, lease or transfer any properties or assets, with certain exceptions; and enter into certain merger, consolidation or other reorganization transactions, with certain exceptions.
A breach of any of these covenants could result in an event of default under our credit facility. Upon the occurrence of an event of default, the lender could elect to declare all amounts outstanding under such facility to be immediately due and payable and terminate all commitments to extend further credit. In addition, the Company would be subject to additional restrictions if an event of default exists under the Credit Agreement, such as a prohibition on the payment of cash dividends.
Our business and financial results can be adversely affected by foreign currency exchange rates, changes in our tax rates and tax liabilities and assessments (including as a result of changes in tax laws).
International markets contribute a substantial portion of our revenues, and we intend to continue expanding our presence in these regions. The exposure to fluctuations in currency exchange rates takes on different forms. International revenues and costs are subject to the risk that fluctuations in exchange rates could adversely affect our reported revenues and profitability when translated into U.S. dollars for financial reporting purposes. These fluctuations could also adversely affect the demand for products and services provided by us. As a multinational corporation, our businesses occasionally invoice third-party customers in currencies other than the one in which they primarily do business (the "functional currency"). Movements in the invoiced currency relative to the functional currency could adversely impact our cash flows and our results of operations. As our international sales grow, exposure to fluctuations in currency exchange rates could have a larger effect on our financial results. In fiscal 2022, currency translation had an unfavorable effect of $12.5 million on revenues due to the strengthening of the U.S. dollar relative to other currencies in which the company sells products and services.
As a global company, we are subject to taxation in numerous countries, states and other jurisdictions. In particular, we are affected by the impact of changes to tax laws or related authoritative interpretations in the United States, including tax reform under the Tax Cuts and Jobs Act which became effective in late 2017, which included broad and complex changes
25
to the United States tax code. Interpretations, assumptions and guidance regarding the Tax Act that have been issued subsequently have had a material impact on our effective tax rate, and we anticipate that there may be additional changes to the U.S. tax code in the future.
In preparing our financial results, we record the amount of tax that is payable in each of the countries, states and other jurisdictions in which we operate. Our future effective tax rate, however, may be lower or higher than experienced in the past due to numerous factors, including a change in the mix of our profitability from country to country, changes in accounting for income taxes and recently enacted and future changes in tax laws in jurisdictions in which we operate. Any of these factors could cause us to experience an effective tax rate significantly different from previous periods or our current expectations, which could have an adverse effect on our business, results of operations and cash flows.
Dividends on our common stock could be reduced or eliminated in the future.
For many years, our Board has declared quarterly dividends. In the future, our Board may determine to reduce or eliminate our common stock dividend in order to fund investments for growth, repurchase shares or conserve capital resources.
Legal, Regulatory, Compliance and Reputational Risks
Our business is subject to extensive regulation; failure to comply with these regulations could adversely affect our business and financial results.
As referenced in more detail above, we and our customers must comply with a wide array of federal, state, local and international regulations, in such areas as medical device, healthcare, import and export, anticorruption, and privacy. We develop, configure and market our products to meet customer needs created by those regulations. Any significant change in regulations could reduce demand for our products or increase our expenses. For example, many of our instruments are marketed to the pharmaceutical industry for use in discovering and developing drugs and diagnostic products. Changes in the U.S. FDA’s regulation of drug or medical device products could have an adverse effect on the demand for these products.
We have agreements relating to the sale of our products to government entities in the U.S. and elsewhere and, as a result, we are subject to various statutes and regulations that apply to companies doing business with the government (approximately 2% of our fiscal 2022 sales were made to the U.S. federal government). The laws governing government contracts differ from the laws governing private contracts and government contracts may contain pricing terms and conditions that are not applicable to private contracts. We are also subject to investigation for compliance with the regulations governing government contracts. A failure to comply with these regulations could result in suspension of these contracts, criminal, civil and administrative penalties or debarment.
We are subject to various local, state, federal, foreign and transnational laws and regulations, which include the operating and security standards of the U.S. FDA, the U.S. Drug Enforcement Agency (the DEA), the U.S. Department of Health and Human Services (the DHHS), and other comparable agencies and, in the future, any changes to such laws and regulations could adversely affect us. In particular, we are subject to laws and regulations concerning current good manufacturing practices. Our subsidiaries may be required to register for permits and/or licenses with, and may be required to comply with the laws and regulations of, the DEA, the FDA, the DHHS, foreign agencies and/or comparable state agencies as well as certain accrediting bodies depending upon the type of operations and location of product distribution, manufacturing and sale. The manufacture, distribution and marketing of many of our products and services, including medical devices and pharma services, are subject to extensive ongoing regulation by the FDA, the DEA, and other equivalent local, state, federal and non-U.S. regulatory authorities. In addition, we are subject to inspections by these regulatory authorities. We are the sole manufacturer of a number of products for many of our customers and a negative regulatory event could impact our customers’ ability to provide products to their customers.
We are also subject to a variety of federal, state, local and international laws and regulations that govern, among other things, the importation and exportation of products, the handling, transportation and manufacture of substances that could be classified as hazardous, and our business practices in the U.S. and abroad such as anti-competition laws. Any noncompliance by us with applicable laws and regulations or the failure to maintain, renew or obtain necessary permits
26
and licenses could result in criminal, civil and administrative penalties and could have an adverse effect on our results of operations.
Significant developments or changes in U.S. laws or policies, including changes in U.S. trade policies and tariffs and the reaction of other countries thereto, can have an adverse effect on our business and financial results.
Significant developments or changes in U.S. laws and policies (including as a result of changes in party control of Congress or decisions from the U.S. Supreme Court), such as laws and policies governing foreign trade, manufacturing, and development and investment in the territories and countries where we or our customers operate, or governing the health care system and drug prices, can adversely affect our business and financial results. For example, the previous U.S. administration increased tariffs on certain goods imported into the United States and trade tensions between the United States and China escalated, with each country imposing significant, additional tariffs on a wide range of goods imported from the other country. That trade tension has not diminished under the current U.S. administration. The U.S. and China could impose other types of restrictions such as limitations on government procurement or technology export restrictions, which could affect our access to markets. These factors have adversely affected, and in the future could further adversely affect, our business and financial results.
Our business and financial results can be impaired by improper conduct by any of our employees, agents or business partners.
We cannot provide assurance that our internal controls and compliance systems, including our Code of Ethics and Business Conduct, protect us from unauthorized acts committed by employees, agents or business partners of ours (or of businesses we acquire or partner with) that violate U.S. and/or non-U.S. laws, including the laws governing payments to government officials, bribery, fraud, kickbacks and false claims, pricing, sales and marketing practices, conflicts of interest, competition, employment practices and workplace behavior, export and import compliance, economic and trade sanctions, money laundering and data privacy. In particular, the U.S. Foreign Corrupt Practices Act, the UK Bribery Act and similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business, and we operate in many parts of the world that have experienced governmental corruption to some degree. Any such improper actions or allegations of such acts could damage our reputation and subject us to civil or criminal investigations in the United States and in other jurisdictions and related shareholder lawsuits, could lead to substantial civil and criminal, monetary and non-monetary penalties and could cause us to incur significant legal and investigatory fees. In addition, the government may seek to hold us liable for violations committed by companies in which we invest or that we acquire. We also rely on our suppliers to adhere to our supplier code of conduct, and material violations of such code of conduct could occur that could have a material effect on our business and financial results.
Certain of our businesses are subject to extensive regulation by the U.S. FDA and by comparable agencies of other countries, as well as laws regulating fraud and abuse in the healthcare industry and the privacy and security of health information. Failure to comply with those regulations could adversely affect our business and financial results.
As stated above, certain of our products are medical devices, diagnostics tests and other products that are subject to regulation by the U.S. FDA or state CLIA regulations, by other federal and state governmental agencies, by comparable agencies of other countries and regions and by regulations governing hazardous materials and drugs-of abuse (or the manufacture and sale of products containing any such materials). The global regulatory environment has become increasingly stringent and unpredictable. Several countries that did not have regulatory requirements for medical devices have established such requirements in recent years, and other countries have expanded, or plan to expand, their existing regulations, including implementation of IVDR regulations in Europe. For example, the EU has adopted the In Vitro Diagnostic Regulation (the “EU IVDR”), which imposes stricter requirements for the marketing and sale of in vitro diagnostic medical devices, including in the area of clinical evaluation requirements, quality systems and post-market surveillance. Manufacturers of in vitro diagnostics medical devices that have been marketed and sold under the prior regulatory regime now have to comply with some of the new EU IVDR requirements, while the effective date of other requirements have been delayed. Complying with EU IVDR, the regulation applicable to the Company, may require material modifications to our quality management systems, additional resources in certain functions, updates to technical files and additional clinical data in some cases, among other changes. Failure by us or by our customers to comply with
27
the requirements of the EU IVDR, or other requirements imposed by these or similar regulatory authorities, including without limitation, remediating any inspectional observations to the satisfaction of these regulatory authorities, could result in warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution, restrictions on our operations, civil or criminal sanctions, or withdrawal of existing or denial of pending approvals, including those relating to products or facilities. In addition, such a failure could expose us to contractual or product liability claims, contractual claims from our customers, including claims for reimbursement for lost or damaged active pharmaceutical ingredients, as well as ongoing remediation and increased compliance costs, any or all of which could be significant. Failure to meet these requirements adversely impacts our business and financial results in the applicable geographies.
Government authorities may conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law. Failure to obtain required regulatory clearances before marketing our products (or before implementing modifications to or promoting additional indications or uses of our products), other violations of laws or regulations, failure to remediate inspectional observations to the satisfaction of these regulatory authorities, real or perceived efficacy or safety concerns or trends of adverse events with respect to our products (even after obtaining clearance for distribution) and unfavorable or inconsistent clinical data from existing or future clinical trials can lead to FDA Form 483 Inspectional Observations, warning letters, notices to customers, declining sales, loss of customers, loss of market share, remediation and increased compliance costs, recalls, seizures of adulterated or misbranded products, fines, expenses, injunctions, civil penalties, criminal penalties, consent decrees, administrative detentions, refusals to permit importations, partial or total shutdown of production facilities or the implementation of operating restrictions, narrowing of permitted uses for a product, refusal of the government to grant 510(k) clearance, suspension or withdrawal of approvals, pre-market notification rescissions and other adverse effects. Further, defending against any such actions can be costly and time-consuming and may require significant personnel resources. Therefore, even if we are successful in defending against any such actions brought against us, our business may be impaired. Ensuring that our internal operations and business arrangements with third parties comply with applicable laws and regulations also involves substantial costs.
More specifically, as a healthcare provider, the Company’s Exosome Diagnostics’ ExoDx Prostate business is subject to extensive regulation at the federal, state, and local levels in the U.S. and other countries where it operates. The Company’s failure to meet governmental requirements under these regulations, including those relating to billing practices and financial relationships with physicians, hospitals, and health systems, could lead to civil and criminal penalties, exclusion from participation in Medicare and Medicaid, and possibly prohibitions or restrictions on the use of its laboratories. While the Company believes that it is in material compliance with all statutory and regulatory requirements, there is a risk that government authorities might take a contrary position. Such occurrences, regardless of their outcome, could damage the Company’s reputation and adversely affect important business relationships it has with third parties.
Failure to comply with privacy and security laws and regulations could result in fines, penalties and damage to the Company’s reputation and have a material adverse effect upon the Company’s business, a risk that has been elevated with the acquisition of Exosome Diagnostics, whose laboratory testing service is a healthcare provider that obtains and uses protected health information.
If the Company does not comply with existing or new laws and regulations related to protecting the privacy and security of personal or health information, it could be subject to monetary fines, civil penalties or criminal sanctions. In the U.S., the Health Insurance Portability and Accountability Act of 1996 (HIPAA) privacy and security regulations, including the expanded requirements under U.S. Health Information Technology for Economic and Clinical Health Act (HITECH), establish comprehensive standards with respect to the use and disclosure of protected health information (PHI) by covered entities, in addition to setting standards to protect the confidentiality, integrity and security of PHI. HIPAA restricts the Company’s ability to use or disclose PHI, without patient authorization, for purposes other than payment, treatment or healthcare operations (as defined by HIPAA), except for disclosures for various public policy purposes and other permitted purposes outlined in the privacy regulations. If the laboratory operations for the Company’s business use or disclose PHI improperly under these privacy regulations, they may incur significant fines and other penalties for wrongful use or disclosure of PHI in violation of the privacy and security regulations, including potential civil and criminal fines and penalties.
28
ITEM 1B. UNRESOLVED STAFF COMMENTS
There are no unresolved staff comments as of the date of this report.
ITEM 2. PROPERTIES
The Company owns the facilities that its headquarters and R&D Systems subsidiary occupy in Minneapolis, Minnesota. The Minneapolis facilities are utilized by both the Company’s Protein Sciences and Diagnostics and Genomics segments.
The Minneapolis complex includes approximately 800,000 square feet of space in several adjoining buildings. Bio-Techne uses approximately 710,000 square feet of the complex for administrative, research, manufacturing, shipping and warehousing activities. The Company is currently leasing the remaining space in the complex as retail and office space. The Company also owns a 61,000 square foot facility in Saint Paul, Minnesota that is utilized for additional manufacturing capabilities and activities.
The Company also owns a 34,000 square foot manufacturing facility in Flowery Branch, Georgia. This facility is utilized by the Company’s Protein Sciences segment.
The Company owns a 17,000 square foot facility that its Bio-Techne Europe subsidiary occupies in Abingdon, England. This facility is utilized by the Company’s Protein Sciences and Diagnostics and Genomics segments.
The Company owns a 9,000 square foot facility that its Canada subsidiaries occupy in Toronto, Canada. This facility is utilized by the Company’s Protein Sciences and Diagnostics and Genomics segments.
The Company owns a 52,700 square foot manufacturing facility in Wallingford, Connecticut. This facility is utilized by the Company’s Protein Sciences segment.
The Company leases the following material facilities, all of which are primarily utilized by the Company’s Protein Sciences segment with the exception of the locations used by the Company’s ProteinSimple and CyVek subsidiaries, which support both the Protein Sciences segment and the Diagnostics & Genomics segment. Certain locations are not named because they were not significant individually or in the aggregate as of the date of this report.
Subsidiary |
| Location |
| Type |
| Square Feet |
Bio-Techne Ltd |
| Langley, United Kingdom |
| Warehouse |
| 14,300 |
Bio-Techne China |
| Shanghai and Beijing, China |
| Office/warehouse |
| 25,500 |
Boston Biochem |
| Cambridge, Massachusetts |
| Office/lab |
| 7,400 |
Tocris |
| Bristol, United Kingdom |
| Office/manufacturing/lab/warehouse |
| 30,000 |
PrimeGene |
| Shanghai, China |
| Office/manufacturing/lab |
| 20,600 |
Bionostics |
| Devens, Massachusetts |
| Office/manufacturing |
| 70,000 |
Novus Biologicals |
| Centennial, Colorado |
| Office/warehouse |
| 29,400 |
ProteinSimple |
| San Jose, California |
| Office/manufacturing/warehouse |
| 98,000 |
ProteinSimple Ltd. |
| Ottawa, Canada |
| Office/manufacturing/warehouse |
| 10,800 |
CyVek |
| Wallingford, Connecticut |
| Office/manufacturing/warehouse |
| 17,500 |
Cliniqa |
| San Marcos, California |
| Office/manufacturing/warehouse |
| 62,800 |
Advanced Cell Diagnostics |
| Newark, California |
| Office/manufacturing/warehouse |
| 55,900 |
Bio-Techne France |
| Rennes, France |
| Office/warehouse |
| 11,000 |
Exosome Diagnostics |
| Waltham, Massachusetts |
| Office/manufacturing/warehouse |
| 38,400 |
R&D Systems |
| Minneapolis, Minnesota |
| Office/manufacturing/warehouse |
| 10,700 |
Asuragen |
| Austin, Texas |
| Office/manufacturing/warehouse |
| 47,400 |
Bio-Techne Ireland | Dublin, Ireland | Warehouse | 25,000 |
The Company entered into a definitive agreement in November 2021 for a 74,000 square foot facility in Centennial, Colorado for the next 12.5 years with annual rental impact of $0.9 million. Construction is underway and once complete,
29
the commencement of the lease will occur, which is expected to be in the first half of fiscal 2023. The facility replaces a current leased facility in the same location that will terminate upon completion of construction of the new facility. The Company believes the owned and leased properties, inclusive of the leased property in Colorado, are adequate to meet its occupancy needs in the foreseeable future.
ITEM 3. LEGAL PROCEEDINGS
As of August 19, 2022, the Company is not a party to any legal proceedings that, individually or in the aggregate, are reasonably expected to have a material adverse effect on the Company’s business, results of operations, financial condition or cash flows.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER
MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
The Company’s common stock is listed on the NASDAQ stock exchange under the symbol “TECH”.
Holders of Common Stock and Dividends Paid
As of August 19, 2022, there were over 121,000 beneficial shareholders of the Company’s common stock and over 148 shareholders of record. The Company paid annual cash dividends totaling $50.2 million, $49.6 million, and $48.9 million in fiscal 2022, 2021, and 2020, respectively. The Board of Directors periodically considers the payment of cash dividends, and there is no guarantee that the Company will pay comparable cash dividends, or any cash dividends, in the future.
In connection with the acquisition of Exosome Diagnostics, Inc. on August 1, 2018, the Company entered into a new credit facility that provides for a revolving credit facility of $600 million, which can be increased by an additional $200 million subject to certain conditions, and a term loan of $250 million. The credit facility is governed by a Credit Agreement dated August 1, 2018 and matures on August 1, 2023. The Credit Agreement that governs the revolving line of credit contains customary events of default and would prohibit payment of dividends to Company shareholders in the event of a default thereunder.
Issuer Purchases of Equity Securities
During the years ended June 30, 2022 and June 30, 2021, the Company repurchased 394,238 shares of its common stock at an average share price of $408.26 and 120,000 shares at an average share price of $359.82, respectively. The Company's previous share repurchase plan, implemented in fiscal 2019, granted management the discretion to mitigate the dilutive effect of stock option exercises for fiscal 2018, which then increases in each period subsequent to June 30, 2018 for additional dilutive impacts of stock options exercised in those future periods. On February 2, 2022, the Company replaced the prior share repurchase plan with a new share repurchase plan that authorizes the Company to purchase up to $400 million in stock. The Company repurchased 89,238 shares for $41.3 million in fiscal 2022 under the previous plan. The Company repurchased 305,000 shares for $119.7 million in fiscal 2022 under the new share repurchase plan. As of June 30, 2022, the Company had $280.3 million available to repurchase under our existing plan.
Stock Performance Graph
The following chart compares the cumulative total shareholder return on the Company’s common stock with the S&P 500 Index, the S&P 500 Life Sciences Tools and Services Index, the S&P Midcap 400 Index and the S&P 400 MidCap Life Sciences Tools and Services Index. The comparison assumes $100 was invested on the last trading day before July 1, 2017 in the Company’s common stock and in each of the foregoing indices and assumes reinvestment of dividends. The
30
Company became part of the S&P 500 Index during fiscal 2022. The S&P 400 Index was included for comparative purposes to the prior year Form 10-K.

31
ITEM 6. SELECTED FINANCIAL DATA
RESERVED
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL
CONDITION AND RESULTS OF OPERATIONS
The following management discussion and analysis (“MD&A”) provides information that we believe is useful in understanding our operating results, cash flows and financial condition. We provide quantitative information about the material sales drivers including the effect of acquisitions and changes in foreign currency at the corporate and segment level. We also provide quantitative information about discrete tax items and other significant factors we believe are useful for understanding our results. The MD&A should be read in conjunction with the consolidated financial information and related notes included in this Form 10-K. This discussion contains various “Non-GAAP Financial Measures” and also contains various “Forward-Looking Statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We refer readers to the statements entitled “Non-GAAP Financial Measures” located at the end of this MD&A and “Forward-Looking Information and Cautionary Statements” and “Risk Factors” within Items 1 and 1A of this Form 10-K.
OVERVIEW
Bio-Techne develops, manufactures and sells life science reagents, instruments and services for the research and clinical diagnostic markets worldwide. With our deep product portfolio and application expertise, we sell integral components of scientific investigations into biological processes and molecular diagnostics, revealing the nature, diagnosis, etiology and progression of specific diseases. Our products aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses.
We manage the business in two operating segments – our Protein Sciences segment and our Diagnostics and Genomics segment. Our Protein Sciences segment is a leading developer and manufacturer of high-quality biological reagents used in all aspects of life science research, diagnostics and cell and gene therapy. This segment also includes proteomic analytical tools, both manual and automated, that offer researchers and pharmaceutical manufacturers efficient and streamlined options for automated western blot and multiplexed ELISA workflow. Our Diagnostics and Genomics segment develops and manufactures diagnostic products, including controls, calibrators, and diagnostic assays for the regulated diagnostics market, exosome-based molecular diagnostic assays, advanced tissue-based in-situ hybridization assays for spatial genomic and tissue biopsy analysis, and genetic and oncology kits for research and clinical applications.
RECENT ACQUISITIONS
A key component of the Company's strategy is to augment internal growth at existing businesses with complementary acquisitions. The Company did not make any acquisitions in fiscal year 2022. As disclosed in Note 1, the Company made a $25 million investment in a forward contract, which allows the Company to acquire Wilson Wolf based on certain revenue or EBITDA thresholds being met. As further disclosed in Note 13, the Company closed on the acquisition of Namocell, Inc on July 1, 2022.
OVERALL RESULTS
Operational Update
For fiscal 2022, consolidated net sales increased 19% as compared to fiscal 2021. Organic growth was 17%, with acquisitions having a favorable impact of 3% and foreign currency translation having an unfavorable impact of 1%. Organic revenue growth was broad based and driven by overall execution of the Company's long-term growth strategy.
Consolidated earnings, including non-controlling interest, increased 88% compared to fiscal 2021. The increase in earnings was driven by non-operating mark-to-market gain of $16 million on our ChemoCentryx investment in fiscal year 2022, compared to a loss on the investment of $67.9 million in the prior fiscal year. Additionally, fiscal year 2022 had adjustments of $20.4 million of benefit related to contingent considerations as compared to a charge of $5.3 million in the prior fiscal year. After adjusting for acquisition related costs, intangibles amortization, stock-based compensation,
32
restructuring costs, the gain on investment, and impact from partially-owned consolidated subsidiaries, adjusted net earnings increased 18% in fiscal 2022 as compared to fiscal 2021. Adjusted earnings growth was primarily driven by sales growth.
For fiscal 2021, consolidated net sales increased 26% as compared to fiscal 2020. Organic growth was 22%, with currency translation and acquisitions having a 3% and 1% impact on revenue respectively. Organic revenue growth was broad based and driven by accelerated momentum of the Company's long-term growth strategy as well as customer site closures in the latter half of fiscal 2020 due to the COVID-19 pandemic.
For fiscal 2021, consolidated earnings, including non-controlling interest, decreased 39% compared to fiscal 2020. The decrease in earnings was primarily due to a non-operating loss of approximately $67.9 million on our ChemoCentryx investment, compared to a gain on investment of $137 million in the last fiscal year. After adjusting for acquisition related costs, intangibles amortization, stock-based compensation, restructuring costs, the loss on investment, certain income tax items in both years, and non-controlling interest, adjusted net earnings increased 52% in fiscal 2021 as compared to fiscal 2020. Adjusted earnings growth was driven by the reopening of customer sites closing during the latter half of fiscal 2020, volume leverage, operational productivity, and product mix.
Business Strategy Update
Environmental
The Company’s key business strategies for long-term growth and profitability continue to be geographic expansion, core product innovation, acquisitions and talent retention and development. The Company was also focused on evaluating how climate change impacts from our business operations might be measured and mitigated, with the plan of integrating consideration of greenhouse gas emissions and other climate variables into those key business strategies.
In response to the COVID-19 pandemic, the Company took additional steps to monitor and strengthen our supply chain to maintain an uninterrupted supply of our critical products and services. The Company has maintained these procedures while incorporating additional considerations regarding potential adverse weather events associated with climate change.
The financial impact of potential environmental regulations pertaining to carbon emissions or the integration of climate change impacts into our core business strategies are not expected to materially alter the Company’s near-term financial results. Additionally, the Company is creating a cross-functional internal council to evaluate potential long-term business impacts while driving long-term sustainability solutions.
Digital
In driving our four key business strategies, the Company utilizes digital networks and systems for data transmission, transaction processing, and storing of electronic information. As disclosed in “Item 1A. Risk Factors”, increased cybersecurity attack activity poses a risk for our business. In response to this risk, the Company actively completes system patching and required maintenance, performs internal and third-party employee training, monitors network and system activity, and completes data backups for our systems. However, even with the Company’s procedures performed, our digital networks and systems are still potentially vulnerable to cyberattacks.
The financial impact of our cybersecurity initiatives and activities are ongoing and not expected to have a material impact on our financial results. However, the impact on our business operations and financial results from a material cyber breach would be unknown and dependent on the nature of the breach.
RESULTS OF OPERATIONS
Net Sales
Consolidated organic net sales exclude the impact of companies acquired during the first 12 months post-acquisition and the effect of the change from the prior year in exchange rates used to convert sales in foreign currencies (primarily the euro, British pound sterling, and Chinese yuan) into U.S. dollars.
33
Consolidated net sales growth was as follows:
| Year Ended June 30, |
| |||||
| 2022 |
| 2021 |
| 2020 |
| |
Organic sales growth |
| 17 | % | 22 | % | 4 | % |
Acquisitions sales growth |
| 3 | % | 1 | % | 0 | % |
Impact of foreign currency fluctuations |
| (1) | % | 3 | % | 0 | % |
Consolidated net sales growth |
| 19 | % | 26 | % | 4 | % |
Consolidated net sales by segment were as follows (in thousands):
| Year Ended June 30, | ||||||||
| 2022 |
| 2021 |
| 2020 | ||||
Protein Sciences | $ | 832,311 | $ | 704,564 | $ | 555,352 | |||
Diagnostics and Genomics |
| 274,843 |
| 227,744 |
| 184,549 | |||
Intersegment |
| (1,555) |
| (1,276) |
| (1,210) | |||
Consolidated net sales | $ | 1,105,599 | $ | 931,032 | $ | 738,691 | |||
In fiscal 2022, Protein Sciences segment net sales increased 18% compared to fiscal 2021. Organic growth for the segment was 19% for the fiscal year, with currency translation having an unfavorable 1% impact on revenue.
Overall segment growth was driven by strong BioPharma demand resulting in broad-based growth across our proteomic research reagents and analytical tools.
In fiscal 2022, Diagnostics and Genomics segment net sales increased 21% compared to fiscal 2021. Organic growth for the segment was 10% with acquisitions contributing 11% and currency translation having an immaterial impact on revenue growth.
Segment growth was driven by the full year impact of the Asuragen acquisition and organic growth. Organic growth was driven by an exclusive agreement entered into for development, finalization and commercialization of our ExoTRU kidney transplant rejection test, and continued strength in our diagnostic reagent product lines.
In fiscal 2021, Protein Sciences segment net sales increased 27% compared to fiscal 2020. Organic growth for the segment was 24% for the fiscal year, with foreign currency translation having a favorable impact of 3%, and acquisitions contributing an immaterial amount.
Overall segment growth was driven by continued market acceptance of our portfolio of productivity enhancing solutions across end-markets and geographies combined with the reopening of customer sites that were closed in the latter half of fiscal 2020 due to COVID-19.
In fiscal 2021, Diagnostics and Genomics segment net sales increased 23% compared to fiscal 2020. Organic growth was 18% with acquisitions and foreign currency having a favorable impact of 4% and 1% impact on revenue, respectively.
Overall segment revenue growth was driven by broad based organic growth across product lines and geographies and the acquisition of Asuragen in the fourth quarter of fiscal year 2021. RNAscope products had an exceptional year in both the Academia and Bio-Pharma end markets, while the Exosome product line also provided year over year growth despite navigating limitations and/or customer avoidance of non-essential medical procedures throughout fiscal 2021 associated with the COVID-19 pandemic.
34
Gross Margins
Consolidated gross margins were 68.4%, 68.0%, and 65.4% in fiscal 2022, 2021, and 2020. Consolidated gross margins were positively impacted as a result of broad based revenue growth. Excluding the impact of acquired inventory sold, amortization of intangibles, stock compensation expense, and the impact of partially-owned consolidated subsidiaries, adjusted gross margins were 72.5%, 72.3%, and 70.3% in fiscal 2022, 2021, and 2020 respectively. Fiscal 2022 adjusted gross margin was positively impacted by volume leverage and product mix, partially offset by additional investments made in the business to support future growth, when compared to fiscal 2020 and fiscal 2019.
A reconciliation of the reported consolidated gross margin percentages, adjusted for acquired inventory sold and intangible amortization included in cost of sales, is as follows:
| ||||||
Year Ended June 30, | ||||||
2022 |
| 2021 |
| 2020 |
| |
Consolidated gross margin percentage | 68.4 | % | 68.0 | % | 65.4 | % |
Identified adjustments: |
|
|
|
| ||
Costs recognized upon sale of acquired inventory | 0.1 | % | 0.2 | % | — | % |
Amortization of intangibles | 3.7 | % | 3.8 | % | 4.7 | % |
Stock compensation expense - COGS | 0.1 | % | 0.2 | % | 0.2 | % |
Impact of partially owned consolidated subsidiaries(1) | 0.2 | % | 0.1 | % | — | % |
Non-GAAP adjusted gross margin percentage | 72.5 | % | 72.3 | % | 70.3 | % |
(1)Adjusted gross margin percentages for fiscal 2021 have been updated for comparability to fiscal 2022 for the inclusion of the impact of partially-owned consolidated subsidiaries on the Company’s adjusted gross margin percentage.
Fluctuations in adjusted gross margins, as a percentage of net sales, have primarily resulted from changes in foreign currency exchange rates and changes in product mix. We expect that, in the future, gross margins will continue to be impacted by the mix of our portfolio growing at different rates as well as future acquisitions.
Management uses adjusted operating results to monitor and evaluate performance of the Company’s two segments. Segment gross margins, as a percentage of net sales, were as follows:
| Year Ended June 30, |
| |||||
2022 |
| 2021 |
| 2020 |
| ||
Protein Sciences |
| 75.5 | % | 76.0 | % | 75.0 | % |
Diagnostics and Genomics |
| 63.1 | % | 60.5 | % | 55.6 | % |
The changes in the Protein Sciences segment’s gross margin percentage for fiscal 2022 as compared to fiscal 2021 and 2020 was primarily attributable to mix of product sales within the segment.
The increase in the Diagnostics and Genomics segment’s gross margin for fiscal 2022 as compared to fiscal 2021 and fiscal 2020 was primarily due to volume leverage.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased $47.8 million (15%) in fiscal 2022 when compared to fiscal 2021. Selling, general, and administrative expenses increased primarily due to the full year impact of prior year’s Asuragen acquisition and strategic investments made in the business to support future growth.
Selling, general and administrative expenses increased $64.4 million (25%) in fiscal 2021 when compared to fiscal 2020. Selling, general, and administrative expenses increased primarily due to investments made by the Company to support volume growth within each of the segments as well as additional expenses related to the acquisition of Asuragen, Inc.
35
Consolidated selling, general and administrative expenses were composed of the following (in thousands):
| Year Ended June 30, | ||||||||
2022 | 2021 | 2020 | |||||||
Protein Sciences | $ | 195,328 | $ | 159,489 | $ | 138,792 | |||
Diagnostics and Genomics |
| 93,578 |
| 75,160 |
| 65,407 | |||
Total segment expenses |
| 288,906 |
| 234,649 |
| 204,199 | |||
Amortization of intangibles |
| 32,492 |
| 27,788 |
| 26,358 | |||
Acquisition related expenses |
| (19,082) |
| 7,097 |
| 415 | |||
Eminence impairment(1) | 18,715 | — | — | ||||||
Gain on escrow litigation |
| — |
| — |
| (7,159) | |||
Restructuring costs |
| 1,640 |
| 142 |
| 87 | |||
Stock-based compensation |
| 45,085 |
| 50,200 |
| 32,667 | |||
Corporate selling, general and administrative expenses |
| 5,010 |
| 5,075 |
| 4,016 | |||
Total selling, general and administrative expenses | $ | 372,766 | $ | 324,951 | $ | 260,583 | |||
(1)Refer to the Goodwill Impairment section within the Critical Accounting Policies for further details on the Eminence impairment.
Research and Development Expenses
Research and development expenses increased $16.5 million (23%) and $5.4 million (8%) in fiscal 2022 and 2021, respectively, as compared to prior year periods. The increase in research and development expenses in fiscal 2022 as compared to 2021 was primarily attributable to strategic growth investments and the Asuragen acquisition in the fourth quarter of fiscal 2021. The increase in research and development expenses in fiscal 2021 as compared to fiscal 2020 was primarily attributable to continued investment in future growth platforms of the Company and recent acquisitions.
| Year Ended June 30, | ||||||||
2022 | 2021 | 2020 | |||||||
Protein Sciences | $ | 56,370 | $ | 46,361 | $ | 43,022 | |||
Diagnostics and Genomics |
| 30,770 |
| 24,242 |
| 22,170 | |||
Total segment expenses |
| 87,140 |
| 70,603 |
| 65,192 | |||
Unallocated corporate expenses |
| — |
| — |
| — | |||
Total research and development expenses | $ | 87,140 | $ | 70,603 | $ | 65,192 | |||
Net Interest Income / (Expense)
Net interest income/(expense) for fiscal 2022, 2021, and 2020 was ($10.5) million, $(13.5) million, and $(18.6) million, respectively. Net interest expense in fiscal 2022 decreased when compared to fiscal 2021 due to a reduction in our average long-term debt, which coincided with a reduction in the notional amount on our variable interest derivative.
Net interest expense in fiscal 2021 decreased when compared to fiscal 2020 due to a reduction in our average long-term debt, which coincided with a reduction in the notional amount on our variable interest derivative.
36
Other Non-Operating Expense, Net
Other non-operating expense, net, consists of foreign currency transaction gains and losses, rental income, building expenses related to rental property and the Company’s gains and losses on investments as follows (in thousands):
| Year Ended June 30, | ||||||||
2022 | 2021 | 2020 | |||||||
Foreign currency gains (losses) | $ | 699 | $ | (6,650) | $ | 1,703 | |||
Rental income |
| 599 |
| 1,036 |
| 1,140 | |||
Real estate taxes, depreciation and utilities |
| (2,035) |
| (1,845) |
| (1,915) | |||
Gain (loss) on investment |
| 15,186 |
| (68,047) |
| 137,508 | |||
Miscellaneous (expense) income |
| 862 |
| (136) |
| (786) | |||
Other non-operating income (expense), net | $ | 15,311 | $ | (75,642) | $ | 137,650 | |||
During fiscal 2022, the Company recognized gains of $16.1 million related to changes in fair value associated with changes in the stock price of our ChemoCentryx, Inc. (CCXI) investment. Additionally, the Company recognized losses of $1.1 million related to changes in fair value associated with changes in the stock price of our exchange traded investment grade bond funds. As described in Note 13, on August 4, 2022, the Company sold all of its shares in CCXI.
During fiscal 2021, the Company recognized losses of $67.9 million related to changes in fair value associated with changes in the stock price of our ChemoCentryx, Inc. (CCXI) investment.
During fiscal 2020, the Company recognized gains of $137.5 million related to changes in fair value associated with changes in the stock price of our ChemoCentryx, Inc. (CCXI) investment.
Income Taxes
Income taxes for fiscal 2022, 2021, and 2020 were at effective rates of 12.7%, 5.8%, and 17.1%, respectively, of consolidated earnings before income taxes. The change in the effective tax rate for fiscal 2022 compared to fiscal 2021 was driven by a mix of increased net income and the dilutive effect the increased net income has on the favorable rate benefits, which are mainly related to share-based compensation. The Company had share-based compensation excess tax benefits of $29.3 million in fiscal 2022. The Company’s discrete tax benefits in fiscal 2021 primarily related to share-based compensation excess tax benefits of $28.1 million. The Company’s discrete tax benefits in fiscal 2020 primarily related to share-based compensation excess tax benefits of $17.7 million.
37
Net Earnings
Non-GAAP adjusted consolidated net earnings and earnings per share are as follows (in thousands):
| |||||||||
Year Ended June 30, | |||||||||
2022 | 2021 | 2020 |
| ||||||
Net earnings before taxes - GAAP | $ | 301,386 | $ | 148,175 | $ | 276,477 | |||
Identified adjustments attributable to Bio-Techne: |
|
|
|
| |||||
Costs recognized upon sale of acquired inventory |
| 1,596 |
| 1,565 |
| — | |||
Amortization of intangibles |
| 73,054 |
| 64,239 |
| 60,865 | |||
Acquisition related expenses |
| (18,694) |
| 7,489 |
| 793 | |||
Gain on escrow settlement |
| — |
| — |
| (7,170) | |||
Eminence impairment | 18,715 | — | |||||||
Stock based compensation, inclusive of employer taxes |
| 46,401 |
| 51,846 |
| 34,262 | |||
Restructuring costs |
| 1,640 |
| 142 |
| 87 | |||
Investment (gain) loss and other |
| (16,171) |
| 68,391 |
| (136,716) | |||
Impact of partially owned subsidiaries(1) |
| 2,675 |
| 1,390 |
| — | |||
Earnings before taxes - Adjusted | $ | 410,602 | $ | 343,237 | $ | 228,598 | |||
Non-GAAP tax rate |
| 21.2 | % |
| 20.2 | % |
| 21.6 | % |
Non-GAAP tax expense |
| 87,090 |
| 69,478 |
| 49,280 | |||
Non-GAAP adjusted net earnings attributable to Bio-Techne(1) | $ | 323,512 |
| 273,759 |
| 179,318 | |||
Earnings per share - diluted - Adjusted |
| 7.89 |
| 6.76 |
| 4.55 | |||
(1)Adjusted consolidated net earnings and earnings per share for fiscal 2021 have been updated for comparability to fiscal 2022 for the inclusion of the impact of partially-owned consolidated subsidiaries on the Company’s adjusted consolidated net earnings and earnings per share.
Depending on the nature of discrete tax items, our reported tax rate may not be consistent on a period to period basis. The Company independently calculates a non-GAAP adjusted tax rate considering the impact of discrete items and jurisdictional mix of the identified non-GAAP adjustments. The following table summarizes the reported GAAP tax rate and the effective Non-GAAP adjusted tax rate for the periods ended June 30, 2022, 2021, and 2020.
| ||||||
Year Ended June 30, | ||||||
2022 | 2021 | 2020 |
| |||
GAAP effective tax rate | 12.7 | % | 5.8 | % | 17.1 | % |
Discrete items | 11.3 |
| 19.0 |
| 7.0 | |
Long-term GAAP tax rate | 24.0 | % | 24.8 | % | 24.1 | |
Rate impact items |
|
|
|
|
| |
Stock based compensation | (1.9) | % | (5.7) | % | (2.4) | % |
Acquisition costs | (0.0) |
| (0.2) |
| 0.4 | |
Change in fair value of investments | (0.1) |
| 0.5 |
| (0.4) | |
Other | (0.8) |
| 0.8 |
| (0.1) | |
Total rate impact items | (2.8) | % | (4.6) | % | (2.5) | % |
Non-GAAP adjusted tax rate(1) | 21.2 | % | 20.2 | % | 21.6 | % |
Refer to Note 11 for additional discussion relating to the change in discrete tax items between fiscal 2022 and fiscal 2021.
38
LIQUIDITY AND CAPITAL RESOURCES
Cash, cash equivalents and available-for-sale investments at June 30, 2022 were $247.0 million compared to $231.6 million at June 30, 2021. Included in available-for-sale investments at June 30, 2022 and June 30, 2021 was the fair value of the Company’s investment in CCXI of $36.0 million and $20.0 million, respectively, as well as the Company’s exchange traded investment grade bond funds of $23.9 million as of June 30, 2022. The Company purchased these bond funds during the year ended June 30, 2022.
At June 30, 2022, approximately 31% of the Company’s cash and equivalent account balances of $172.6 million were located in the U.S., with the remainder located in primarily in Canada, China, the U.K. and other European countries.
At June 30, 2022, approximately 48% of the Company’s available-for-sale investment account balances of $74.5 million were located in the U.S., with the remaining 32% in Canada and 20% in China.
The Company has either paid U.S. taxes on its undistributed foreign earnings or intends to indefinitely reinvest the undistributed earnings in the foreign operations or expects the earnings will be remitted in a tax neutral transaction. Management of the Company expects to be able to meet its cash and working capital requirements for operations, facility expansion, capital additions, and cash dividends for the foreseeable future, and at least the next 12 months, through currently available funds, including funds available through our line-of-credit and cash generated from operations.
Future acquisition strategies may or may not require additional borrowings under the line-of-credit facility or other outside sources of funding.
Cash Flows From Operating Activities
The Company generated cash from operations of $325.3 million, $352.2 million, and $205.2 million in fiscal 2022, 2021, and 2020 respectively. The decrease in cash generated from operating activities in fiscal 2022 as compared to fiscal 2021 was mainly a result of changes in the timing of cash payments on certain operating assets and liabilities, largely offset by an increase in year over year net earnings. The increase in cash generated from operating activities in fiscal 2021 as compared to fiscal 2020 was mainly a result of an increase in year over year operating income of $79.9 million and a $29.3 million benefit to operating cash from year-over-year changes in operating assets and liabilities as well as a non-cash stock-based compensation expense of $16.6 million.
Cash Flows From Investing Activities
We continue to make investments in our business, including capital expenditures. There are no cash payments for acquisitions during fiscal year 2022. The Company acquired Eminence Biotechnology and Asuragen, Inc. during fiscal year 2021 for a total of approximately $225.4 million, net of cash acquired. The Company did not make any acquisitions in fiscal 2020.
The Company’s net proceeds (outflow) from the purchase, sale and maturity of available-for-sale investments in fiscal 2022, 2021, and 2020 were $(26.9) million, $26.7 million, and $76.9 million, respectively. The decrease in fiscal 2022 compared to fiscal 2021 was driven by the purchase of the exchange traded investment grade bond funds, which have a cost basis of $25.0 million. The decrease in fiscal 2021 compared to fiscal 2020 was driven by the sale of a portion of the CCXI investment in fiscal year 2020, which did not reoccur in fiscal year 2021. The Company’s investment policy is to place excess cash in certificates of deposit with the objective of obtaining the highest possible return while minimizing risk and keeping the funds accessible.
Capital additions in fiscal year 2022, 2021, and 2020 were $44.9 million, $44.3 million, and $51.7 million. Fiscal 2022 capital expenditures related to investments in new buildings, machinery, and IT equipment. Fiscal 2021 capital expenditures related to investments in new buildings, in particular, the Company’s GMP manufacturing facility. Capital additions planned for fiscal 2023 are approximately $62 million and are expected to be financed through currently available cash and cash generated from operations. Increase in expected additions in fiscal 2023 is related to increasing capacity to meet expected sales growth across the Company.
39
During the year ended June 30, 2022, the Company paid $25 million to enter into a two-part forward contract which requires the Company to purchase the full equity interest in Wilson Wolf Corporation (Wilson Wolf) if certain annual revenue or EBITDA thresholds are met. The Company is currently forecasting the first option payment of $231 million to occur in fiscal 2023 with the second option payment of approximately $1 billion plus potential contingent consideration occurring between fiscal 2026 and fiscal 2028.
Cash Flows From Financing Activities
In fiscal 2022, 2021, and 2020, the Company paid cash dividends of $50.2 million, $49.6 million, $48.9 million, respectively. The Board of Directors periodically considers the payment of cash dividends.
The Company received $77.2 million, $65.1 million, $71.0 million, for the exercise of options for 613,000, 627,000, 743,000 shares of common stock in fiscal 2022, 2021 and 2020, respectively.
During fiscal 2022, 2021, and 2020, the Company repurchased $161.0 million, $43.2 million, and $50.1 million, respectively, in share repurchases included as a cash outflow within Financing Activities.
During fiscal 2022, 2021, and 2020, the Company drew $90.0 million, $256.0 million, and $40.0 million, respectively, under its revolving line-of-credit facility. Repayments of $175.5 million, $271.5 million, and $188.5 million were made on its line-of-credit in fiscal 2022, 2021, and 2020, respectively.
During fiscal 2022, the Company made $4.0 million in cash payments towards the Quad contingent consideration liability. Of the $4.0 million in total payments, $0.7 million is classified as financing on the statement of cash flows. The remaining $3.3 million is recorded as operating on the statement of cash flows as it represents the consideration liability that exceeds the amount of the contingent consideration liability recognized at the acquisition date. During fiscal 2021, there were no payments related to contingent consideration classified as financing activities. The Company made $0.3 million in contingent consideration payments, which were classified within operating activities. During fiscal 2020, the Company made $4.4 million ($4 million for Quad and $0.4 million for B-MoGen) in cash payments towards the Quad, Exosome, and B-MoGen contingent consideration liabilities. Of the $4.4 million in total payments, $3.4 million is classified as financing on the statement of cash flows. The remaining $1 million is recorded as operating on the statement of cash flows.
During fiscal 2022, 2021 and 2020, the Company paid $23.5 million, $19.3 million and $3.8 million, respectively, for taxes remitted on behalf of participants in net share settlement transactions and restricted stock units. This is included as a cash outflow within the other financing activities line of the consolidated statements of cash flows.
CRITICAL ACCOUNTING POLICIES
Management’s discussion and analysis of the Company’s financial condition and results of operations are based upon the Company’s Consolidated Financial Statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP). The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, management evaluates its estimates. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
The Company has identified the policies outlined below as critical to its business operations and an understanding of results of operations. The listing is not intended to be a comprehensive list of all accounting policies; investors should also refer to Note 1 to the Consolidated Financial Statements included in Item 8 of this Annual Report on Form 10-K.
Business Combinations
We allocate the purchase price of acquired businesses to the estimated fair values of the assets acquired and liabilities assumed as of the date of the acquisition. The calculations used to determine the fair value of the long-lived assets acquired,
40
primarily intangible assets, can be complex and require significant judgment. We weigh many factors when completing these estimates including, but not limited to, the nature of the acquired company’s business; its competitive position, strengths, and challenges; its historical financial position and performance; estimated customer retention rates; discount rates; and future plans for the combined entity. We may also engage independent valuation specialists, when necessary, to assist in the fair value calculations for significant acquired long-lived assets.
The fair value of acquired technology is generally the primary asset identified and therefore estimated using the multi-period excess earnings method. The multi-period excess earnings method model estimates revenues and cash flows derived from the primary asset and then deducts portions of the cash flow that can be attributed to supporting assets, such as Trade Names and in-process research and development, that contributed to the generation of the cash flows. The resulting cash flow, which is attributable solely to the primary asset acquired, is then discounted at a rate of return commensurate with the risk of the asset to calculate a present value. The Trade Name is generally calculated using the relief from royalty method, which calculates the cost savings associated with owning rather than licensing the technology. Assumed royalty rates are applied to the projected revenues for the remaining useful life of the technology to estimate the royalty savings. In-process research and development assets are valued using the multi-period excess earnings method when the cash flows from the in-process research and development assets are separately identifiable from the primary asset. In circumstances that Customer Relationship assets are identified that are not the primary asset, they are valued using the distributor model income approach, which isolates revenues and cash flow associated with the sales and distribution function of the entity and attributable to customer-related assets, which are then discounted at a rate of return commensurate with the risk of the asset to calculate a present value.
We estimate the fair value of liabilities for contingent consideration by discounting to present value the probability weighted contingent payments expected to be made. For potential payments related to financial performance based milestones, projected revenue and/or EBITDA amounts, volatility and discount rates assumptions are included in the estimated amounts. For potential payments related to product development milestones, the fair value is based on the probability of achievement of such milestones. The excess of the purchase price over the estimated fair value of the net assets acquired is recorded as goodwill. Goodwill is not amortized, but is subject to impairment testing on at least an annual basis.
We are also required to estimate the useful lives of the acquired intangible assets, which determines the amount of acquisition-related amortization expense we will record in future periods. Each reporting period, we evaluate the remaining useful lives of our amortizable intangibles to determine whether events or circumstances warrant a revision to the remaining period of amortization.
While we use our best estimates and assumptions, our fair value estimates are inherently uncertain and subject to refinement. As a result, during the measurement period, which may be up to one year from the acquisition date, we may record adjustments to the assets acquired and liabilities assumed, with the corresponding offset to goodwill. Any adjustments required after the measurement period are recorded in the consolidated statements of earnings.
The judgments required in determining the estimated fair values and expected useful lives assigned to each class of assets and liabilities acquired can significantly affect net income. For example, different classes of assets will have useful lives that differ. Consequently, to the extent a longer-lived asset is ascribed greater value than a shorter-lived asset, net income in a given period may be higher. Additionally, assigning a lower value to amortizable intangibles would result in a higher amount assigned to goodwill. As goodwill is not amortized, this would benefit net income in a given period, although goodwill is subject to annual impairment analysis.
Impairment of Goodwill
Goodwill
Goodwill was $822.1 million as of June 30, 2022, which represented 36% of total assets. Goodwill is tested for impairment on an annual basis in the fourth quarter of each year, or more frequently if events occur or circumstances change that could indicate a possible impairment.
41
To analyze goodwill for impairment, we must assign our goodwill to individual reporting units. Identification of reporting units includes an analysis of the components that comprise each of our operating segments, which considers, among other things, the manner in which we operate our business and the availability of discrete financial information. Components of an operating segment are aggregated to form one reporting unit if the components have similar economic characteristics. We periodically review our reporting units to ensure that they continue to reflect the manner in which we operate our business.
In the first quarter of fiscal 2022, the Company combined the management of the Exosome Diagnostics and Asuragen reporting units, both of which are included in the Diagnostics and Genomics operating segment. In conjunction with the combination of the reporting units, a qualitative goodwill impairment assessment was performed. The qualitative assessment identified no indicators of impairment.
In the second quarter of fiscal 2022, Changzhou Eminence Biotechnology Co., Ltd. (Eminence) notified the Company of its need for additional capital to execute its growth plan. The Company first attempted to find outside equity financing support for the Eminence investment but was unable to do so. The Company then reviewed the additional financing needs required to successfully ramp Eminence’s business, which ultimately did not meet the Company’s return on capital requirements. Therefore, the Company did not provide additional funding to Eminence. As a result of not obtaining additional financing, Eminence notified the Company of its plans to cease operations and liquidate its business.
Given the upcoming liquidation process to dispose of the Eminence assets, the Company identified a triggering event and performed impairment testing during the second quarter of fiscal 2022. The impairment testing resulted in a full impairment of the Eminence goodwill and intangible assets, which resulted in charges of $8.3 million and $8.6 million, respectively, for the year ended June 30, 2022. The Company also recognized inventory and fixed asset impairment charges of $0.9 million and $0.9 million, respectively. The Company recorded the impairment charges within the General and Administrative line in the Consolidated Income Statement. The impairment charges recorded within Net Earnings Attributable to Bio-Techne were reduced by approximately $8 million recorded within Net Earnings Attributable to Noncontrolling Interests. The remaining net tangible assets of Eminence included in our Consolidated Balance Sheet as of June 30 2022, were $4.3 million and primarily consisted of fixed assets and related deposits of $3.1 million, inventory of $0.6 million, receivables of $0.4 million, and other current assets of $0.1 million. The Company also had $4.5 million related to current liabilities. The Company holds a financial interest of approximately 57.4% in those tangible assets in the upcoming liquidation process.
2022 Goodwill Impairment Analyses
In completing our 2022 annual goodwill impairment analyses, we elected to perform a quantitative assessment for all five of our reporting units. A quantitative assessment involves comparing the carrying value of the reporting unit, including goodwill, to its estimated fair value. Carrying value is based on the assets and liabilities associated with the operations of the reporting unit, which often requires the allocation of shared or corporate items among reporting units. In accordance with ASU 2017‑04, a goodwill impairment charge is recorded for the amount by which the carrying value of a reporting unit exceeds the fair value of the reporting unit. In determining the fair values of our reporting units, we utilized the income approach. The income approach is a valuation technique under which we estimated future cash flows using the reporting unit’s financial forecast from the perspective of an unrelated market participant. Using historical trending and internal forecasting techniques, we projected revenue and applied our fixed and variable cost experience rates to the projected revenue to arrive at the future cash flows. A terminal value was then applied to the projected cash flow stream. Future estimated cash flows were discounted to their present value to calculate the estimated fair value. The discount rate used was the value- weighted average of our estimated cost of capital derived using both known and estimated customary market metrics. In determining the estimated fair value of a reporting unit, we were required to estimate a number of factors, including projected operating results, terminal growth rates, economic conditions, anticipated future cash flows, the discount rate and the allocation of shared or corporate items.
The result of our quantitative assessment indicated that all of the reporting units had a substantial amount of headroom as of April 1, 2022. This impairment assessment is sensitive to changes in forecasted cash flows, as well as our selected discount rate. Changes in the reporting unit’s results, forecast assumptions and estimates could materially affect the estimation of the fair value of the reporting units. The Company did not identify any triggering events after our annual
42
goodwill impairment through June 30, 2022, the date of our consolidated balance sheet, that would require an additional goodwill impairment assessment to be performed.
2021 Goodwill Impairment Analyses
In completing our 2021 annual goodwill impairment analyses, we elected to perform a quantitative assessment for each of our five reporting units. A quantitative assessment involves comparing the carrying value of the reporting unit, including goodwill, to its estimated fair value. Carrying value is based on the assets and liabilities associated with the operations of the reporting unit, which often requires the allocation of shared or corporate items among reporting units. In accordance with ASU 2017-04, a goodwill impairment charge is recorded for the amount by which the carrying value of a reporting unit exceeds the fair value of the reporting unit. In determining the fair values of our reporting units, we utilized the income approach. The income approach is a valuation technique under which we estimated future cash flows using the reporting unit’s financial forecast from the perspective of an unrelated market participant. Using historical trending and internal forecasting techniques, we projected revenue and applied our fixed and variable cost experience rates to the projected revenue to arrive at the future cash flows. A terminal value was then applied to the projected cash flow stream. Future estimated cash flows were discounted to their present value to calculate the estimated fair value. The discount rate used was the value-weighted average of our estimated cost of capital derived using both known and estimated customary market metrics. In determining the estimated fair value of a reporting unit, we were required to estimate a number of factors, including projected operating results, terminal growth rates, economic conditions, anticipated future cash flows, the discount rate and the allocation of shared or corporate items.
Because our 2021 quantitative analyses included all of our reporting units, the summation of our reporting units’ fair values, as indicated by our discounted cash flow calculations, were compared to our consolidated fair value, as indicated by our market capitalization, to evaluate the reasonableness of our calculations. This impairment assessment is sensitive to changes in forecasted cash flows, as well as our selected discount rate. Changes in the reporting unit’s results, forecast assumptions and estimates could materially affect the estimation of the fair value of the reporting units.
The quantitative assessment completed as of April 1, 2021 indicated that all of the reporting units had a substantial amount of headroom. Accordingly, the Company determined there was no indication of impairment of goodwill in our annual goodwill impairment analysis. Further, no triggering events were identified in the year ended June 30, 2021 that would require an additional goodwill impairment assessment beyond our required annual goodwill impairment assessment.
2020 Goodwill Impairment Analyses
In completing our 2020 annual goodwill impairment analyses, we elected to perform a quantitative assessment for all of our reporting units. A quantitative assessment involves comparing the carrying value of the reporting unit, including goodwill, to its estimated fair value. Carrying value is based on the assets and liabilities associated with the operations of the reporting unit, which often requires the allocation of shared or corporate items among reporting units. In accordance with ASU 2017-04, a goodwill impairment charge is recorded for the amount by which the carrying value of a reporting unit exceeds the fair value of the reporting unit. In determining the fair values of our reporting units, we utilized the income approach. The income approach is a valuation technique under which we estimated future cash flows using the reporting unit’s financial forecast from the perspective of an unrelated market participant. Using historical trending and internal forecasting techniques, we projected revenue and applied our fixed and variable cost experience rates to the projected revenue to arrive at the future cash flows. A terminal value was then applied to the projected cash flow stream. Future estimated cash flows were discounted to their present value to calculate the estimated fair value. The discount rate used was the value-weighted average of our estimated cost of capital derived using both known and estimated customary market metrics. In determining the estimated fair value of a reporting unit, we were required to estimate a number of factors, including projected operating results, terminal growth rates, economic conditions, anticipated future cash flows, the discount rate and the allocation of shared or corporate items.
Because our 2020 quantitative analyses included all of our reporting units, the summation of our reporting units’ fair values, as indicated by our discounted cash flow calculations, were compared to our consolidated fair value, as indicated by our market capitalization, to evaluate the reasonableness of our calculations. This impairment assessment is sensitive to changes in forecasted cash flows, as well as our selected discount rate. Changes in the reporting unit’s results, forecast assumptions and estimates could materially affect the estimation of the fair value of the reporting units.
43
The quantitative assessment completed as of April 1, 2020 indicated that all of the reporting units had a substantial amount of headroom. Accordingly, the Company determined there was no indication of impairment of goodwill in our annual goodwill impairment analysis. Further, no triggering events were identified in the year ended June 30, 2020 that would require an additional goodwill impairment assessment beyond our required annual goodwill impairment assessment.
NEW ACCOUNTING PRONOUNCEMENTS
Information regarding the accounting policies adopted during fiscal 2022 and those not yet adopted can be found under caption “Note 1: Description of Business and Summary of Significant Accounting Policies” of the Notes to the Consolidated Financial Statements appear in Item 8 of this report.
SUBSEQUENT EVENTS
On July 1, 2022, the Company completed the acquisition of Namocell, Inc. for approximately $100 million, plus contingent consideration of up to $25 million upon the achievement of certain future milestones.
On August 4, 2022, the Company sold its remaining shares of CCXI for approximately $73.3 million. The cost basis of the investment was $6.6 million.
NON-GAAP FINANCIAL MEASURES
This Annual Report on Form 10-K, including “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7, contains financial measures that have not been calculated in accordance with accounting principles generally accepted in the U.S. (GAAP). These non-GAAP measures include:
| ● | Organic growth |
| ● | Adjusted gross margin |
| ● | Adjusted operating margin |
| ● | Adjusted net earnings |
| ● | Adjusted effective tax rate |
We provide these measures as additional information regarding our operating results. We use these non-GAAP measures internally to evaluate our performance and in making financial and operational decisions, including with respect to incentive compensation. We believe that our presentation of these measures provides investors with greater transparency with respect to our results of operations and that these measures are useful for period-to-period comparison of results.
Our non-GAAP financial measure of organic growth represents revenue growth excluding revenue from acquisitions within the preceding 12 months, the impact of foreign currency, as well as the impact of partially-owned consolidated subsidiaries. Excluding these measures provides more useful period-to-period comparison of revenue results as it excludes the impact of foreign currency exchange rates, which can vary significantly from period to period, and revenue from acquisitions that would not be included in the comparable prior period. Revenue from partially-owned subsidiaries consolidated in our financial statements are also excluded from our organic revenue calculation, as those revenues are not fully attributable to the Company. Revenue from partially-owned subsidiaries was $4.6 million for the year ended June 30, 2022.
44
Our non-GAAP financial measures for adjusted gross margin, adjusted operating margin, and adjusted net earnings, in total and on a per share basis, exclude stock-based compensation, the costs recognized upon the sale of acquired inventory, amortization of acquisition intangibles, acquisition related expenses inclusive of the changes in fair value of contingent consideration, and other non-recurring items including non-recurring costs, goodwill and long-lived asset impairments, and gains. Stock-based compensation is excluded from non-GAAP adjusted net earnings because of the nature of this charge, specifically the varying available valuation methodologies, subjection assumptions, variety of award types, and unpredictability of amount and timing of employer related tax obligations. The Company excludes amortization of purchased intangible assets, purchase accounting adjustments, including costs recognized upon the sale of acquired inventory and acquisition-related expenses inclusive of the changes in fair value contingent consideration, and other non-recurring items including gains or losses on legal settlements, goodwill and long-lived asset impairment charges, and one-time assessments from this measure because they occur as a result of specific events, and are not reflective of our internal investments, the costs of developing, producing, supporting and selling our products, and the other ongoing costs to support our operating structure. Additionally, these amounts can vary significantly from period to period based on current activity. The Company also excludes revenue and expense attributable to partially-owned consolidated subsidiaries in the calculation of our non-GAAP financial measures as the revenues and expenses are not fully attributable to the Company.
The Company’s non-GAAP adjusted operating margin and adjusted net earnings, in total and on a per share basis, also excludes stock-based compensation expense, which is inclusive of the employer portion of payroll taxes on those stock awards, restructuring, impairments of equity method investments, gain and losses from investments, and certain adjustments to income tax expense. Impairments of equity investments are excluded as they are not part of our day-to-day operating decisions. Additionally, gains and losses from other investments that are either isolated or cannot be expected to occur again with any predictability are excluded. Costs related to restructuring activities, including reducing overhead and consolidating facilities, are excluded because we believe they are not indicative of our normal operating costs. The Company independently calculates a non-GAAP adjusted tax rate to be applied to the identified non-GAAP adjustments considering the impact of discrete items on these adjustments and the jurisdictional mix of the adjustments. In addition, the tax impact of other discrete and non-recurring charges which impact our reported GAAP tax rate are adjusted from net earnings. We believe these tax items can significantly affect the period-over-period assessment of operating results and not necessarily reflect costs and/or income associated with historical trends and future results.
The Company periodically reassesses the components of our non-GAAP adjustments for changes in how we evaluate our performance, changes in how we make financial and operational decisions, and considers the use of these measures by our competitors and peers to ensure the adjustments are still relevant and meaningful.
Readers are encouraged to review the reconciliations of the adjusted financial measures used in management’s discussion and analysis of the financial condition of the Company to their most directly comparable GAAP financial measures provided within the Company’s consolidated financial statements.
45
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES
ABOUT MARKET RISK
The Company operates internationally, and thus is subject to potentially adverse movements in foreign currency exchange rates. Approximately 34% of the Company’s consolidated net sales in fiscal 2022 were made in foreign currencies, including 12% in euro, 4% in British pound sterling, 7% in Chinese yuan and the remaining 11% in other currencies. The Company is exposed to market risk primarily from foreign exchange rate fluctuations of the euro, British pound sterling, Chinese yuan and Canadian dollar as compared to the U.S. dollar as the financial position and operating results of the Company’s foreign operations are translated into U.S. dollars for consolidation.
Month-end exchange rates between the euro, British pound sterling, Chinese yuan, Canadian dollar and the U.S. dollar, which have not been weighted for actual sales volume in the applicable months in the periods, were as follows:
Year Ended June 30, | ||||||||
2022 | 2021 | 2020 | ||||||
Euro |
|
|
|
|
| |||
High | $ | 1.19 | $ | 1.23 | $ | 1.12 | ||
Low |
| 1.05 |
| 1.16 |
| 1.09 | ||
Average |
| 1.12 |
| 1.20 |
| 1.11 | ||
British pound sterling |
|
|
|
|
| |||
High | $ | 1.39 | $ | 1.42 | $ | 1.32 | ||
Low |
| 1.21 |
| 1.29 |
| 1.22 | ||
Average |
| 1.32 |
| 1.35 |
| 1.26 | ||
Chinese yuan |
|
|
|
|
| |||
High | $ | 0.16 | $ | 0.16 | $ | 0.15 | ||
Low |
| 0.15 |
| 0.14 |
| 0.14 | ||
Average |
| 0.15 |
| 0.15 |
| 0.14 | ||
Canadian dollar |
|
|
|
|
| |||
High | $ | 0.81 | $ | 0.83 | $ | 0.77 | ||
Low |
| 0.78 |
| 0.75 |
| 0.71 | ||
Average |
| 0.79 |
| 0.78 |
| 0.74 | ||
The Company’s exposure to foreign exchange rate fluctuations also arises from trade receivables and intercompany payables denominated in one currency in the financial statements, but receivable or payable in another currency.
The Company does not enter into foreign currency forward contracts to reduce its exposure to foreign currency rate changes on forecasted intercompany sales transactions or on intercompany foreign currency denominated balance sheet positions. Foreign currency transaction gains and losses are included in "Other non-operating expense, net" in the Consolidated Statement of Earnings and Comprehensive Income. The effect of translating net assets of foreign subsidiaries into U.S. dollars are recorded on the Consolidated Balance Sheet as part of "Accumulated other comprehensive income (loss)."
The effects of a hypothetical simultaneous 10% appreciation in the U.S. dollar from June 30, 2022 levels against the euro, British pound sterling, Chinese yuan and Canadian dollar are as follows (in thousands):
Decrease in translation of earnings of foreign subsidiaries (annualized) |
| $ | 4,618 |
Decrease in translation of net assets of foreign subsidiaries |
| 74,218 | |
Additional transaction losses |
| 3,177 |
46
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
CONSOLIDATED STATEMENTS OF EARNINGS AND COMPREHENSIVE INCOME
Bio-Techne Corporation and Subsidiaries
(in thousands, except per share data)
Year Ended June 30, | ||||||||
2022 | 2021 | 2020 | ||||||
Net sales | $ | | $ | | $ | | ||
Cost of sales |
| |
| |
| | ||
Gross margin |
| |
| |
| | ||
Operating expenses: |
|
|
|
|
|
| ||
Selling, general and administrative |
| |
| |
| | ||
Research and development |
| |
| |
| | ||
Total operating expenses |
| |
| |
| | ||
Operating income |
| |
| |
| | ||
Other income (expense) |
|
|
| |||||
Interest expense |
| ( |
| ( |
| ( | ||
Interest income |
| |
| |
| | ||
Other non-operating income (expense), net |
| |
| ( |
| | ||
Total other income (expense), net |
| |
| ( |
| | ||
Earnings before income taxes |
| |
| |
| | ||
Income taxes (benefit) |
| |
| |
| | ||
Net earnings, including noncontrolling interest |
| |
| |
| | ||
Net earnings (loss) attributable to noncontrolling interest |
| ( |
| ( |
| — | ||
Net earnings attributable to Bio-Techne | $ | | $ | | $ | | ||
Other comprehensive income (loss): |
|
|
|
|
|
| ||
Foreign currency translation adjustments |
| ( |
| |
| ( | ||
Unrealized gains (losses) on derivative instruments - cash flow hedges, net of tax amounts disclosed in Note 8 |
| |
| |
| ( | ||
Other comprehensive income (loss) |
| ( |
| |
| ( | ||
Other comprehensive income (loss) attributable to noncontrolling interest |
| ( |
| |
| — | ||
Other comprehensive income (loss) attributable to Bio-Techne |
| ( |
| |
| ( | ||
Comprehensive income attributable to Bio-Techne | $ | | $ | | $ | | ||
Earnings per share attributable to Bio-Techne: | ||||||||
Basic | $ | | $ | | $ | | ||
Diluted | $ | | $ | | $ | | ||
Weighted average common shares outstanding: |
|
|
|
|
|
| ||
Basic |
| |
| |
| | ||
Diluted |
| |
| |
| | ||
See Notes to Consolidated Financial Statements.
47
CONSOLIDATED BALANCE SHEETS
Bio-Techne Corporation and Subsidiaries
(in thousands, except share and per share data)
June 30, | |||||
2022 | 2021 | ||||
ASSETS |
|
|
| ||
Current assets: |
|
|
| ||
Cash and cash equivalents | $ | | $ | | |
Short-term available-for-sale investments |
| |
| | |
Accounts receivable, less allowance for doubtful accounts of $ |
| |
| | |
Inventories |
| |
| | |
Other current assets |
| |
| | |
Total current assets |
| |
| | |
Property and equipment, net |
| |
| | |
Right of use asset |
| |
| | |
Goodwill |
| |
| | |
Intangible assets, net |
| |
| | |
Other assets |
| |
| | |
Total assets | $ | | $ | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
| |
Current liabilities: |
|
|
|
| |
Trade accounts payable | $ | | $ | | |
Salaries, wages and related accruals |
| |
| | |
Accrued expenses |
| |
| | |
Contract liabilities |
| |
| | |
Income taxes payable |
| |
| | |
Operating lease liabilities - current |
| |
| | |
Contingent consideration payable |
| — |
| | |
Current portion of long-term debt obligations |
| |
| | |
Other current liabilities |
| |
| | |
Total current liabilities |
| |
| | |
Deferred income taxes |
| |
| | |
Long-term debt obligations |
| |
| | |
Long-term contingent consideration payable |
| |
| | |
Operating lease liabilities |
| |
| | |
Other long-term liabilities |
| |
| | |
Bio-Techne’s Shareholders’ equity: |
|
|
|
| |
Undesignated capital stock, |
| |
| | |
Common stock, par value $ |
| |
| | |
Additional paid-in capital |
| |
| | |
Retained earnings |
| |
| | |
Accumulated other comprehensive loss |
| ( |
| ( | |
Total Bio-Techne’s shareholders’ equity |
| |
| | |
Noncontrolling interest |
| ( |
| | |
Total shareholders’ equity |
| |
| | |
Total liabilities and shareholders’ equity | $ | | $ | | |
See Notes to Consolidated Financial Statements.
48
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
Bio-Techne Corporation and Subsidiaries
(in thousands)
|
|
|
|
| Accumulated |
|
|
| ||||||||||||
Additional | Other | |||||||||||||||||||
Common Stock | Paid-in | Retained | Comprehensive | Noncontrolling | ||||||||||||||||
Shares | Amount | Capital | Earnings | Income(Loss) | Interest | Total | ||||||||||||||
Balances at June 30, 2019 |
| | $ | | $ | | $ | | $ | ( | $ | — | $ | | ||||||
Cumulative effect adjustments due to adoption of new accounting standards and other |
|
|
|
|
| ( |
|
|
|
|
| ( | ||||||||
Net earnings |
|
|
|
|
| |
|
|
|
|
| | ||||||||
Other comprehensive income (loss) |
|
|
|
|
|
| ( |
|
|
| ( | |||||||||
Share repurchases |
| ( |
| ( |
|
| ( |
|
|
|
|
| ( | |||||||
Surrender and retirement of stock to exercise option | ( |
| — |
| ( |
|
|
|
|
|
| ( | ||||||||
Common stock issued for exercise of options |
| |
| |
| |
| ( |
|
|
|
| | |||||||
Common stock issued for restricted stock awards |
| |
| |
| ( |
| ( |
|
|
|
| ( | |||||||
Cash dividends |
|
|
|
| ( |
|
|
|
| ( | ||||||||||
Stock-based compensation expense |
|
| |
|
|
|
|
|
| | ||||||||||
Common stock issued to employee stock purchase plan |
| |
| — |
| |
|
|
|
|
|
| | |||||||
Employee stock purchase plan expense |
|
| |
|
|
|
|
|
| | ||||||||||
Balances at June 30, 2020 |
| | $ | | $ | | $ | | $ | ( | $ | — | $ | | ||||||
Cumulative effect adjustments due to adoption of new accounting standards and other |
|
|
|
| ( |
|
|
|
| ( | ||||||||||
Non-controlling interest in Eminence |
|
|
|
|
|
|
| |
| | ||||||||||
Net earnings |
|
| |
| ( |
| | |||||||||||||
Other comprehensive income (loss) |
|
|
|
| |
| |
| | |||||||||||
Share repurchases |
| ( |
| ( |
|
| ( |
|
|
|
|
| ( | |||||||
Common stock issued for exercise of options |
| |
| |
| |
| ( |
|
|
|
| | |||||||
Common stock issued for restricted stock awards |
| |
|
|
| ( |
|
|
|
| ( | |||||||||
Cash dividends |
|
|
|
| ( |
|
|
|
| ( | ||||||||||
Stock-based compensation expense |
|
| |
|
|
|
|
|
| | ||||||||||
Common stock issued to employee stock purchase plan |
| |
|
| |
|
|
|
|
|
| | ||||||||
Employee stock purchase plan expense |
|
| |
|
|
|
|
|
| | ||||||||||
Balances at June 30, 2021 |
| | $ | | $ | | $ | | $ | ( | $ | | $ | | ||||||
Non-controlling interest in Eminence |
| — | ||||||||||||||||||
Net earnings |
| ( | | |||||||||||||||||
Other comprehensive income (loss) |
| ( | ( | ( | ||||||||||||||||
Share repurchases |
| ( | ( | ( | ( | |||||||||||||||
Common stock issued for exercise of options |
| | ( | | ||||||||||||||||
Common stock issued for restricted stock awards |
| | ( | ( | ||||||||||||||||
Cash dividends |
| ( | ( | |||||||||||||||||
Stock-based compensation expense |
| | ||||||||||||||||||
Common stock issued to employee stock purchase plan |
| | | |||||||||||||||||
Employee stock purchase plan expense |
| | ||||||||||||||||||
Balances at June 30, 2022 |
| | $ | | $ | | $ | | $ | ( | $ | ( | $ | | ||||||
See Notes to Consolidated Financial Statements.
49
CONSOLIDATED STATEMENTS OF CASH FLOWS
Bio-Techne Corporation and Subsidiaries
(in thousands)
Year Ended June 30, | ||||||
2022 | 2021 | 2020 | ||||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
| |||
Net earnings, including noncontrolling interest | $ | | $ | | $ | |
Adjustments to reconcile net earnings to net cash provided by operating activities: |
|
|
|
|
| |
Depreciation and amortization |
| |
| |
| |
Costs recognized on sale of acquired inventory |
| |
| |
| — |
Deferred income taxes |
| |
| ( |
| |
Stock-based compensation expense |
| |
| |
| |
Fair value adjustment to contingent consideration payable |
| ( |
| |
| ( |
Contingent consideration payments - operating |
| ( |
| ( |
| ( |
Fair value adjustment on available for sale investments |
| ( |
| |
| ( |
Asset impairment restructuring | | — | — | |||
Eminence impairment | | — | — | |||
Leases, net |
| ( |
| |
| |
Gain on escrow settlement |
| — |
| — |
| ( |
Other operating activity |
| |
| ( |
| ( |
Change in operating assets and operating liabilities, net of acquisition: |
|
|
|
|
| |
Trade accounts and other receivables, net |
| ( |
| ( |
| |
Inventories |
| ( |
| ( |
| ( |
Prepaid expenses |
| ( |
| ( |
| ( |
Trade accounts payable, accrued expenses, contract liabilities, and other |
| |
| |
| |
Salaries, wages and related accruals |
| |
| |
| |
Income taxes payable |
| |
| |
| ( |
Net cash provided by (used in) operating activities |
| |
| |
| |
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
Proceeds from maturities of available-for-sale investments |
| |
| |
| |
Purchases of available-for-sale investments |
| ( |
| ( |
| ( |
Additions to property and equipment |
| ( |
| ( |
| ( |
Acquisitions, net of cash acquired |
| — |
| ( |
| — |
Investment in unconsolidated entity, net | — | ( |
| | ||
Investment of forward purchase contract |
| ( |
| — | — | |
Net cash provided by (used in) investing activities |
| ( |
| ( |
| |
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
Cash dividends |
| ( |
| ( |
| ( |
Proceeds from stock option exercises |
| |
| |
| |
Re-purchases of common stock |
| ( |
| ( |
| ( |
Borrowings under line-of-credit agreement |
| |
| |
| |
Payments on line-of-credit |
| ( |
| ( |
| ( |
Contingent consideration payments - financing |
| ( |
| — |
| ( |
Taxes paid on RSUs and net share settlements | ( | ( | ( | |||
Other financing activity |
| |
| — |
| — |
Net cash provided by (used in) financing activities |
| ( |
| ( |
| ( |
Effect of exchange rate changes on cash and cash equivalents |
| ( |
| |
| ( |
Net change in cash and cash equivalents |
| ( |
| |
| |
Cash and cash equivalents at beginning of period |
| |
| |
| |
Cash and cash equivalents at end of period | $ | | $ | | $ | |
See Notes to Consolidated Financial Statements.
50
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Bio-Techne Corporation and Subsidiaries
Years ended June 30, 2022, 2021 and 2020
Note 1. Description of Business and Summary of Significant Accounting Policies:
Description of business: Bio-Techne and its subsidiaries, collectively doing business as Bio-Techne Corporation (the Company), develop, manufacture and sell life science reagents, instruments and services for the research and clinical diagnostic markets worldwide. With our deep product portfolio and application expertise, we sell integral components of scientific investigations into biological processes and molecular diagnostics, revealing the nature, diagnosis, etiology and progression of specific diseases. Our products aid in drug discovery efforts and provide the means for accurate clinical tests and diagnoses.
Use of estimates: The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosures of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. These estimates include the valuation of accounts receivable, available-for-sale investments, inventory, intangible assets, contingent consideration, stock-based compensation and income taxes. Actual results could differ from these estimates.
Principles of consolidation: The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All intercompany accounts and transactions have been eliminated. As Changzhou Eminence Biotechnology Co., Ltd. (“Eminence”) met the criteria for consolidation, the transaction was accounted for in accordance with ASC 805, Business Combinations. In applying ASC 805 to the transaction, the Company has elected to include Eminence in our consolidated financial statements on a one month lag.
Translation of foreign financial statements: Assets and liabilities of the Company’s foreign operations are translated at year-end rates of exchange and the resulting gains and losses arising from the translation of net assets located outside the U.S. are recorded as other comprehensive income (loss) on the consolidated statements of earnings and comprehensive income. The cumulative translation adjustment is a component of accumulated other comprehensive loss on the consolidated balance sheets. Foreign statements of earnings are translated at the average rate of exchange for the year. Foreign currency transaction gains and losses are included in other non-operating expense in the consolidated statements of earnings and comprehensive income.
Revenue recognition: ASC 606 provides revenue recognition guidance for any entity that enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of non-financial assets, unless those contracts are within the scope of other accounting standards. The core principle of ASC 606 is that revenue should be recognized to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. Refer to Note 2 for additional information regarding our revenue recognition policy under ASC 606.
Research and development: Research and development expenditures are expensed as incurred. Development activities generally relate to creating new products, improving or creating variations of existing products, or modifying existing products to meet new applications.
Advertising costs: Advertising expenses were $
Income taxes: The Company uses the asset and liability method of accounting for income taxes. Deferred tax assets and liabilities are recognized to record the income tax effect of temporary differences between the tax basis and financial reporting basis of assets and liabilities. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Tax positions taken or expected to be taken in a tax return are recognized in the financial statements when it is more likely than not that the position would be sustained upon examination by tax authorities. A recognized tax
51
position is then measured at the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement. The Company recognizes interest and penalties related to unrecognized tax benefits in income tax expense. Refer to Note 11 for additional information regarding income taxes.
Comprehensive income: Comprehensive income includes charges and credits to shareholders’ equity that are not the result of transactions with shareholders. Our total comprehensive income consists of net income, unrealized gains and losses on cash flow hedges, and foreign currency translation adjustments. The items of comprehensive income, with the exception of net income, are included in accumulated other comprehensive loss in the consolidated balance sheets and statements of shareholders’ equity.
Cash and cash equivalents: Cash and cash equivalents include cash on hand and highly-liquid investments with original maturities of three months or less.
Available-for-sale investments: Available-for-sale investments consist of debt instruments with original maturities of generally three months to six months and equity securities. Available-for-sale investments are recorded based on trade-date. The Company considers all of its marketable securities available-for-sale and reports them at fair value. Unrealized gains and losses on our available-for-sale securities are included within other income (expense) in accordance with ASU 2018-02, which the Company adopted on July 1, 2018.
Trade accounts receivable and allowances: Trade accounts receivable are initially recorded at the invoiced amount upon the sale of goods or services to customers, and they do not bear interest. They are stated net of allowances for doubtful accounts, which represent estimated losses resulting from the inability of customers to make the required payments. The Company adopted ASU 2016-13 on July 1, 2020, which reflects the expected credit losses on financial instruments within its scope, including trade receivables. When determining the allowances for doubtful accounts, we take several factors into consideration, including the overall composition of accounts receivable aging, our prior history of accounts receivable write-offs, the type of customer and our day-to-day knowledge of specific customers. Changes in the allowances for doubtful accounts are included in selling, general and administrative (SG&A) expense in our consolidated statements of earnings and comprehensive income. The point at which uncollected accounts are written off varies by type of customer. The Company does not have material long-term customer receivables. Refer to the Recently Adopted Accounting Pronouncements section of Note 1 for further details.
Inventories: Inventories are stated at the lower of cost (first-in, first-out method) or net realizable value. The Company regularly reviews inventory on hand for slow-moving and obsolete inventory, inventory not meeting quality control standards and inventory subject to expiration.
For certain proteins, antibodies, and chemically based manufactured products, the Company produces larger batches of established products than current sales requirements due to economies of scale through a highly controlled manufacturing process. Accordingly, the manufacturing process for these products has and will continue to produce quantities in excess of forecasted usage. The Company forecasts usage for its products based on several factors including historical demand, current market dynamics, and technological advances. The Company forecasts product usage on an individual product level for a period that is consistent with our ability to reasonably forecast inventory usage for that product. There have been no material changes to the Company’s estimates of the net realizable value for excess and obsolete inventory or other types of inventory reserves and inventory cost adjustments in the fiscal years presented. Additionally, current and historical reserves recorded to reduce the cost of inventory to its net realizable value become part of the new cost basis for the inventory item in accordance with ASC 330 - Inventory.
Property and equipment: Property and equipment are recorded at cost. Equipment is depreciated using the straight-line method over an estimated useful life of
Contingent Consideration: Contingent Consideration relates to the potential payment for an acquisition that is contingent upon the achievement of the acquired business meeting certain product development milestones and/or certain financial performance milestones. The Company records contingent consideration at fair value at the date of acquisition based on the consideration expected to be transferred. For potential payments related to financial performance milestones, we use a real option model in calculating the fair value of the contingent consideration
52
liabilities. The assumptions utilized in the calculation based on financial performance milestones include projected revenue and/or EBITDA amounts, volatility and discount rates. For potential payments related to product development milestones, we estimated the fair value based on the probability of achievement of such milestones. The assumptions utilized in the calculation of the acquisition date fair value include probability of success and the discount rates. Contingent consideration involves certain assumptions requiring significant judgment and actual results may differ from assumed and estimated amounts. Contingent consideration is remeasured each reporting period, and subsequent changes in fair value, including accretion for the passage of time, are recognized within selling, general and administrative in the consolidated statement of earnings and comprehensive income.
Intangible assets: Intangible assets are stated at historical cost less accumulated amortization. Amortization expense is generally determined on the straight-line basis over periods ranging from
Given the anticipated liquidation process to dispose of the Eminence assets, the Company identified a triggering event in the second quarter of fiscal 2022 and performed impairment testing. The impairment testing resulted in a full impairment of the Eminence intangible assets. Refer to the Impairment of Goodwill section as part of Note 1 for further details related to the triggering event and related impairment recorded.
In conjunction with the Asuragen acquisition that occurred in fiscal year 2021, the Company reassessed the useful life of a tradename from a previous acquisition due to the planned integration and cobranding strategy developed with the most recent transaction. As a result, the Company accelerated the amortization of the trade name to be consistent with the life used for the Asuragen trade name. The accelerated amortization resulted in a $
In fiscal year 2020, the Company accelerated the amortization of a certain trade name based on the Company’s planned integration of the products under that acquired trade name into a legacy brand. The accelerated amortization resulted in $
Impairment of long-lived assets and amortizable intangibles: We evaluate the recoverability of property, plant, equipment and amortizable intangibles whenever events or changes in circumstances indicate that an asset’s carrying amount may not be recoverable. Such circumstances could include, but are not limited to, (1) a significant decrease in the market value of an asset, (2) a significant adverse change in the extent or manner in which an asset is used or in its physical condition, or (3) an accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of an asset. We compare the carrying amount of the asset to the estimated undiscounted future cash flows associated with it. If the sum of the expected future net cash flows is less than the carrying value of the asset being evaluated, an impairment loss would be recognized. The impairment loss would be calculated as the amount by which the carrying value of the asset exceeds the fair value of the asset. As quoted market prices are not available for the majority of our assets, the estimate of fair value is based on various valuation techniques, including the discounted value of estimated future cash flows.
The evaluation of asset impairment requires us to make assumptions about future cash flows over the life of the asset being evaluated. These assumptions require significant judgment and actual results may differ from assumed and estimated amounts. No other triggering events were identified and
Impairment of goodwill and indefinite-lived intangible assets: We evaluate the carrying value of goodwill and indefinite-lived intangible assets during the fourth quarter each year and between annual evaluations if events occur or circumstances change that would indicate a possible impairment. Such circumstances could include, but are not limited to, (1) a significant adverse change in legal factors or in business climate, (2) unanticipated competition, (3) an adverse action or assessment by a regulator, or (4) an adverse change in market conditions that are indicative of a decline in the fair value of the assets.
During the fourth quarter of fiscal 2022, the Company performed a qualitative assessment of the acquired in-process research and development assets to determine whether changes in events, circumstances, or the probability of successful
53
development and commercialization of the assets indicated that it is more likely than not that the fair value of the acquired assets are less than its carrying amount. Based on the analysis, the Company determined there was no indication of impairment of the indefinite-lived intangible assets.
To analyze goodwill, we must assign our goodwill to individual reporting units. Identification of reporting units includes an analysis of the components that comprise each of our operating segments, which considers, among other things, the manner in which we operate our business and the availability of discrete financial information. Components of an operating segment are aggregated to form one reporting unit if the components have similar economic characteristics. We periodically review our reporting units to ensure that they continue to reflect the manner in which we operate our business. The Company had
In the first quarter of fiscal 2022, the Company combined the management of the Exosome Diagnostics and Asuragen reporting units, both of which are included in the Diagnostics and Genomics operating segment. In conjunction with the combination of the reporting units, a qualitative goodwill impairment assessment was performed. The qualitative assessment identified no indicators of impairment.
In the second quarter of fiscal 2022, Eminence notified the Company of its need for additional capital to execute its growth plan. The Company first attempted to find outside equity financing support for the Eminence investment but was unable to do so. The Company then reviewed the additional financing needs required to successfully ramp Eminence’s business, which ultimately did not meet the Company’s return on capital requirements. Therefore, the Company did not provide additional funding to Eminence. As a result of not obtaining additional financing, Eminence notified the Company of its plans to cease operations and liquidate its business.
Given the anticipated liquidation process to dispose of the Eminence assets, the Company identified a triggering event and performed impairment testing during the second quarter of fiscal 2022. The impairment testing resulted in a full impairment of the Eminence goodwill and intangible assets, which resulted in charges of $
2022 Goodwill Impairment Analyses
In completing our 2022 annual goodwill impairment analyses, we elected to perform a quantitative assessment for all of our reporting units. A quantitative assessment involves comparing the carrying value of the reporting unit, including goodwill, to its estimated fair value. Carrying value is based on the assets and liabilities associated with the operations of the reporting unit, which often requires the allocation of shared or corporate items among reporting units. In accordance with ASU 2017‑04, a goodwill impairment charge is recorded for the amount by which the carrying value of a reporting unit exceeds the fair value of the reporting unit. In determining the fair values of our reporting units, we utilized the income approach. The income approach is a valuation technique under which we estimated future cash flows using the reporting unit’s financial forecast from the perspective of an unrelated market participant. Using historical trending and internal forecasting techniques, we projected revenue and applied our fixed and variable cost experience rates to the projected revenue to arrive at the future cash flows. A terminal value was then applied to the projected cash flow stream. Future estimated cash flows were discounted to their present value to calculate the estimated fair value. The discount rate used was the value- weighted average of our estimated cost of capital derived using both known and estimated customary market metrics. In determining the estimated fair value of a reporting unit, we were required to estimate a number of factors, including projected operating results, terminal growth rates, economic conditions, anticipated future cash flows, the discount rate and the allocation of shared or corporate items.
54
The result of our quantitative assessment indicated that all of the reporting units had a substantial amount of headroom as of April 1, 2022. This impairment assessment is sensitive to changes in forecasted cash flows, as well as our selected discount rate. Changes in the reporting unit’s results, forecast assumptions and estimates could materially affect the estimation of the fair value of the reporting units. The Company did not identify any triggering events after our annual goodwill impairment through June 30, 2022, the date of our consolidated balance sheet, that would require an additional goodwill impairment assessment to be performed.
2021 Goodwill Impairment Analyses
In completing our 2021 annual goodwill impairment analyses, we elected to perform a quantitative assessment for all of our reporting units. A quantitative assessment involves comparing the carrying value of the reporting unit, including goodwill, to its estimated fair value. Carrying value is based on the assets and liabilities associated with the operations of the reporting unit, which often requires the allocation of shared or corporate items among reporting units. In accordance with ASU 2017-04, a goodwill impairment charge is recorded for the amount by which the carrying value of a reporting unit exceeds the fair value of the reporting unit. In determining the fair values of our reporting units, we utilized the income approach. The income approach is a valuation technique under which we estimated future cash flows using the reporting unit’s financial forecast from the perspective of an unrelated market participant. Using historical trending and internal forecasting techniques, we projected revenue and applied our fixed and variable cost experience rates to the projected revenue to arrive at the future cash flows. A terminal value was then applied to the projected cash flow stream. Future estimated cash flows were discounted to their present value to calculate the estimated fair value. The discount rate used was the value- weighted average of our estimated cost of capital derived using both known and estimated customary market metrics. In determining the estimated fair value of a reporting unit, we were required to estimate a number of factors, including projected operating results, terminal growth rates, economic conditions, anticipated future cash flows, the discount rate and the allocation of shared or corporate items.
The result of our quantitative assessment indicated that all of the reporting units had a substantial amount of headroom as of April 1, 2021. This impairment assessment is sensitive to changes in forecasted cash flows, as well as our selected discount rate. Changes in the reporting unit’s results, forecast assumptions and estimates could materially affect the estimation of the fair value of the reporting units. The Company did not identify any triggering events after our annual goodwill impairment through June 30, 2021, the date of our consolidated balance sheet, that would require an additional goodwill impairment assessment to be performed.
2020 Goodwill Impairment Analyses
The Company elected to perform a quantitative assessment for all of our reporting units in our 2020 goodwill impairment analysis. The quantitative assessment completed utilized a consistent process and methodology to the 2021 goodwill impairment assessment. The result of our quantitative assessment, where we compared the discounted cash flows of each reporting unit to its carrying value, indicated that all of the reporting units had a substantial amount of headroom as of April 1, 2020. This impairment assessment is sensitive to changes in forecasted cash flows, as well as our selected discount rate. Changes in the reporting unit’s results, forecast assumptions and estimates could materially affect the estimation of the fair value of the reporting units. The Company did not identify any triggering events after our annual goodwill impairment through June 30, 2020, the date of our consolidated balance sheet, that would require an additional goodwill impairment assessment to be performed.
Investments: In December 2021, the Company paid $
The first part of the forward contract is triggered upon Wilson Wolf achieving approximately $
55
Once the first part of the forward contract is triggered, the second part of the forward contract will automatically trigger, and requires the Company to acquire the remaining equity interest in Wilson Wolf on December 31, 2027 based on a revenue multiple of approximately
Restructuring actions: Restructuring actions generally include significant actions involving employee-related severance charges, contract termination costs, and impairments and disposals of assets associated with such actions. Employee-related severance charges are based upon distributed employment policies and substantive severance plans. These charges are reflected in the quarter when the actions are probable and the amounts are estimable, which typically is when management approves the associated actions. Asset impairment and disposal charges include right of use assets, leasehold improvements, and other asset write-downs associated with combining operations and disposal of assets.
In September 2021, the Company informed employees of our decision to close our Exosome Diagnostics Germany facility, discontinuing lab and research occurring at the site, as part of a realignment of activities within our Exosome Diagnostics business. The restructuring activities were complete as of June 30, 2022. As a result of the restructuring activities, a pre-tax charge of $
Employee | Asset | ||||||||
| Severance |
| Impairment and other |
| Total | ||||
Selling, general and administrative | $ | | $ | | $ | | |||
Employee | Asset | ||||||||
| Severance |
| Impairment and other |
| Total | ||||
Expense incurred in the first quarter of 2022 | $ | | $ | | $ | | |||
Incremental expense incurred during fiscal 2022 | — | | | ||||||
Cash payments | ( | ( | ( | ||||||
Adjustments(1) | ( | ( | ( | ||||||
Accrued restructuring actions balances as of June 30, 2022 | $ | — | — | — | |||||
(1) Adjustments include refinements to our estimated close down costs as well as the impacts from foreign currency exchange.
During the second quarter of fiscal 2022, the Company also incurred a restructuring charge of $
56
Other Significant Accounting Policies
The following table includes a reference to additional significant accounting policies that are described in other notes to the financial statements, including the note number:
Policy |
| Note |
| |
Fair value measurements |
|
| 5 |
|
Leases | 7 | |||
Earnings per share |
|
| 9 |
|
Share-based compensation |
|
| 10 |
|
Operating segments |
|
| 12 |
|
Recently Adopted Accounting Pronouncements
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326), Measurement of Credit Losses on Financial Instruments. The amendment in this update replaced the previous incurred loss impairment methodology with a methodology that reflects expected credit losses on financial instruments within its scope, including trade and loan receivables and available-for-sale debt securities. This update is intended to provide financial statement users with more decision-useful information about the expected credit losses. The Company adopted this standard on July 1, 2020 using a modified retrospective transition approach with a cumulative impact of $
In August 2018, the FASB issued ASU No. 2018-15, Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract. The standard aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The accounting for the service element of a hosting arrangement that is a service contract is not affected by the new standard. The Company adopted this standard on a prospective basis on July 1, 2020. Accordingly, as of July 1, 2020, the Company records eligible costs to be capitalized within prepaid assets or other non-current assets depending on the nature of the duration of the asset.
In March 2020, the FASB issued ASU No. 2020-04, Facilitation of the Effects of Reference Rate Reform on Financial Reporting and in January 2021 issued ASU No. 2021-01, Reference Rate Reform (Topic 848): Scope. These ASUs provide expedients and exceptions to existing guidance on contract modifications and hedge accounting that is optional to facilitate the market transition from a reference rate, including LIBOR which is being phased out in 2021, to a new reference rate. The provisions of the ASUs impact contract modifications and other changes that occur while LIBOR is phased out. The Company adopted the optional relief guidance provided within these ASUs in the fourth quarter of fiscal 2021 and continues to monitor its debt and derivative instruments that utilize LIBOR as the reference rate. The adoption of the standard did not impact our financial results for fiscal 2022.
Note 2. Revenue Recognition:
Consumables revenues consist of single-use products and are recognized at a point in time following the transfer of control of such products to the customer, which generally occurs upon shipment. Instruments revenues typically consist of longer-lived assets that, for the substantial majority of sales, are recognized at a point in time in a manner similar to consumables. Service revenues consist of extended warranty contracts, post contract support (“PCS”), and custom development projects that are recognized over time as either the customers receive and consume the benefits of such services simultaneously or the underlying asset being developed has no alternative use for the Company at contract inception and the Company has an enforceable right to payment for the portion of the performance completed. Service revenues also include laboratory services recognized at a point in time. Prior to fiscal year 2021, the Company has not recognized revenue upon completion of the performance obligation for laboratory services, but rather upon cash receipt, which was subsequent to the performance obligation being satisfied. The Company accounted for these services based on cash receipts as we did not have significant historical experience collecting payments from Medicare or other insurance providers and considered the variable consideration for such services to be constrained as it would not be probable that a significant amount of revenue
57
would not need to be reversed in future periods for the services provided. Given Medicare coverage for our laboratory services became effective on December 1, 2019, the Company considered that it had sufficient data to estimate variable consideration as of July 1, 2020 for laboratory services that are reimbursed by Medicare. The amount of cash received in fiscal year 2021 for laboratory services reimbursed by Medicare that were performed prior to July 1, 2020 was approximately $
The Company elected the exemption to not disclose the unfulfilled performance obligations for contracts with an original length of one year or less and the exemption to exclude future performance obligations that are accounted under the sales-based or usage-based royalty guidance. The Company’s unfulfilled performance obligations for contracts with an original length greater than one year were not material as of June 30, 2022 and June 30, 2021.
Contracts with customers that contain instruments may include multiple performance obligations. For these contracts, the Company allocates the contract’s transaction price to each performance obligation on a relative standalone selling price basis. Allocation of the transaction price is determined at the contracts’ inception.
Payment terms for shipments to end-users are generally net 30 days. Payment terms for distributor shipments may range from 30 to 90 days. Service arrangements commonly call for payments in advance of performing the work (e.g. extended warranty and service contracts), upon completion of the service (e.g. custom development manufacturing) or a mix of both.
Contract assets include revenues recognized in advance of billings. Contract assets are included within other current assets in the accompanying balance sheet as the amount of time expected to lapse until the company’s right to consideration becomes unconditional is less than one year. We elected the practical expedient allowing us to expense contract costs that would otherwise be capitalized and amortized over the contract period. Contract assets as of June 30, 2022 are not material.
Contract liabilities include billings in excess of revenues recognized, such as those resulting from customer advances and deposits and unearned revenue on warranty contracts. Contract liabilities as of June 30, 2022 and June 30, 2021 were approximately $
Any claims for credit or return of goods must be made within 10 days of receipt. Revenues are reduced to reflect estimated credits and returns. Although the amounts recorded for these revenue deductions are dependent on estimates and assumptions, historically our adjustments to actual results have not been material.
Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from revenue. Amounts billed to customers for shipping and handling are included in revenue, while the related shipping and handling costs are reflected in cost of products. We have elected the practical expedient that allows us to account for shipping and handling activities that occur after the customer has obtained control of a good as a fulfillment cost, and we accrue costs of shipping and handling when the related revenue is recognized.
The following tables present our disaggregated revenue for the periods presented.
Revenue by type is as follows:
Year ended June 30, | ||||||||
2022 |
| 2021 |
| 2020 | ||||
Consumables | $ | | $ | | $ | | ||
Instruments |
| |
| |
| | ||
Services |
| |
| |
| | ||
Total product and services revenue, net |
| | $ | |
| | ||
Royalty revenues |
| |
| |
| | ||
Total revenues, net | $ | | $ | | $ | | ||
58
Revenue by geography is as follows:
Year Ended June 30, | ||||||||
2022 |
| 2021 |
| 2020 | ||||
|
|
|
|
| ||||
United States | $ | | $ | | $ | | ||
EMEA, excluding United Kingdom |
| |
| |
| | ||
United Kingdom |
| |
| |
| | ||
APAC, excluding Greater China |
| |
| |
| | ||
Greater China |
| |
| |
| | ||
Rest of World |
| |
| |
| | ||
Net Sales | $ | | $ | | $ | | ||
Note 3. Supplemental Balance Sheet and Cash Flow Information:
Inventories:
Inventories consist of (in thousands):
June 30, | |||||
2022 |
| 2021 | |||
Raw materials | $ | | $ | | |
Finished goods(1) |
| |
| | |
Inventories, net | $ | | $ | | |
| (1) |
Property and Equipment:
Property and equipment consist of (in thousands):
June 30, | |||||
2022 |
| 2021 | |||
Cost: |
|
|
| ||
Land | $ | | $ | | |
Buildings and improvements |
| |
| | |
Machinery and equipment | |
| | ||
Construction in progress |
| | | ||
Property and equipment, cost |
| |
| | |
Accumulated depreciation and amortization |
| ( |
| ( | |
Property and equipment, net | $ | | $ | | |
59
Intangibles assets were comprised of the following (in thousands):
Useful Life | June 30, | |||||||
(years) | 2022 | 2021 | ||||||
Developed technology |
| 9 - 15 | $ | | $ | | ||
Trade names |
| 2 - 20 |
| |
| | ||
Customer relationships |
| 7 - 16 |
| |
| | ||
Patents |
|
| |
| | |||
Other intangibles |
| 5 - 15 |
| |
| | ||
Definite-lived intangible assets |
| |
| | ||||
Accumulated amortization |
| ( |
| ( | ||||
Definite-lived intangibles assets, net |
| |
| | ||||
In process research and development |
| |
| | ||||
Total intangible assets, net | $ | | $ | | ||||
Changes to the carrying amount of net intangible assets consist of (in thousands):
| June 30, | |||||
2022 | 2021 | |||||
Beginning balance | $ | | $ | | ||
Acquisitions |
| — |
| | ||
Other additions |
| |
| | ||
Amortization expense |
| ( |
| ( | ||
Currency translation | ( | | ||||
Eminence impairment (1) |
| ( |
| — | ||
Ending balance | $ | | $ | | ||
(1) As disclosed in Note 1, the Company recorded an impairment charge of $
Amortization expense related to developed technologies included in cost of sales was $
The estimated future amortization expense for intangible assets as of June 30, 2022, excluding any possible future amortization associated with acquired IPR&D which has not met technological feasibility, is as follows (in thousands):
2023 |
| $ | |
2024 |
| | |
2025 |
| | |
2026 |
| | |
2027 |
| | |
Thereafter |
| | |
Total | $ | |
60
Changes in goodwill by segment and in total consist of (in thousands):
|
| Diagnostics and |
| ||||||
Protein Sciences | Genomics | Total | |||||||
June 30, 2020 |
| $ | |
| $ | |
| $ | |
Acquisitions (Note 4) |
| |
| |
| | |||
Currency translation |
| |
| |
| | |||
June 30, 2021 | $ | | $ | | $ | | |||
Acquisitions(1) |
| — | ( | ( | |||||
Eminence impairment | ( | — | ( | ||||||
Currency translation |
| ( | ( | ( | |||||
June 30, 2022 | $ | | $ | | $ | | |||
(1)As discussed in Note 4, there was an adjustment to the preliminary allocation of the Asuragen acquisition opening balance sheet during the measurement period.
Supplemental Cash Flow Information:
Supplemental cash flow information was as follows (in thousands):
| Year Ended June 30, | ||||||||
| 2022 |
| 2021 |
| 2020 | ||||
Income taxes paid | $ | | $ | | $ | | |||
Interest paid |
| |
| |
| | |||
Non-cash activities: |
|
|
|
|
| ||||
Acquisition-related liabilities(1) |
| |
| |
| ( | |||
Other intangibles(2) |
| — |
| |
| — | |||
| (1) |
| (2) |
Note 4. Acquisitions:
We periodically complete business combinations that align with our business strategy. Acquisitions are accounted for using the acquisition method of accounting, which requires, among other things, that assets acquired and liabilities assumed be recognized at fair value as of the acquisition date and that the results of operations of each acquired business be included in our consolidated statements of comprehensive income from their respective dates of acquisitions. Acquisition costs are recorded in selling, general and administrative expenses as incurred.
There were no acquisitions in fiscal 2022 or fiscal 2020.
61
2021 Acquisitions
Eminence Biotechnology
On October 20, 2020, the Company acquired
As Eminence met the criteria for consolidation, the transaction was accounted for in accordance with ASC 805, Business Combinations. In applying ASC 805 to the transaction, the Company has elected to include Eminence in our consolidated financial statements on a one month lag.
The goodwill recorded as result of the acquisition represents the strategic benefits of growing the Company’s product portfolio and the expected revenue growth from increased market penetration. The fair value of the noncontrolling interest in Eminence was calculated utilizing cash flow projections discounted to the acquisition date and control premiums calculated using market data. Acquired goodwill is not deductible for income tax purposes. The business became part of the Protein Sciences reportable segment in the second quarter of fiscal year 2021. Purchase accounting was finalized during fiscal 2021.
Tangible assets and liabilities acquired were recorded at fair value on the date of close based on management’s assessment. The purchase price allocated to developed technology and customer relationships was based on management’s forecasted cash inflows and outflows and using a multiperiod excess earnings method to calculate the fair value of assets purchased. The amount recorded for developed technology is being amortized with the expense reflected in cost of goods sold in the Condensed Consolidated Statement of Earnings and Comprehensive Income. The amortization period for developed technology is estimated to be
The Company identified a triggering event related to Eminence during the second quarter of fiscal 2022. Refer to Note 1 for further details relating to the triggering event and related impairment recorded.
Asuragen, Inc.
On April 6, 2021, the Company acquired all of the ownership interests of Asuragen, Inc. (Asuragen) for approximately $
Tangible assets and liabilities acquired were recorded at fair value on the date of close based on management's assessment. The purchase price allocated to developed technology, in-process research and development, and customer relationships was based on management's forecasted cash inflows and outflows and using a multiperiod excess earnings method to calculate the fair value of assets purchased. The amount recorded for developed technology is being amortized with the expense reflected in cost of goods sold in the Condensed Consolidated Statement of Earnings and Comprehensive Income.
62
The amortization period for developed technology is estimated to be
The aggregate purchase price of the acquisitions was allocated to the assets acquired and liabilities assumed based on their fair values as of the acquisition date. The following table summarizes the fair values of the assets acquired and liabilities assumed for the fiscal year 2021 acquisitions (in thousands):
Asuragen | Eminence | ||||
Current assets, net of cash | $ | | $ | | |
Equipment and other long-term assets |
| | | ||
Intangible assets: |
| ||||
Developed technology |
| | | ||
In-process research and development |
| | — | ||
Customer relationships |
| | | ||
Trade names |
| | — | ||
Non-competition agreement |
| | — | ||
Goodwill |
| | | ||
Total assets acquired |
| | | ||
Liabilities |
| | | ||
Deferred income taxes, net |
| | | ||
Net assets acquired | $ | | $ | | |
Cash paid, net of cash acquired |
| | | ||
Contingent consideration payable |
| | | ||
Net assets acquired | $ | | $ | | |
Note 5. Fair Value Measurements:
The Company’s financial instruments include cash and cash equivalents, available for sale investments, accounts receivable, accounts payable, contingent consideration obligations, derivative instruments, and long-term debt.
Fair value is defined as the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. This standard also establishes a hierarchy for inputs used in measuring fair value. This standard maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability based on market data obtained from independent sources. Unobservable inputs are inputs that reflect our assumptions about the factors market participants would use in valuing the asset or liability based upon the best information available in the circumstances.
The categorization of financial assets and liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The hierarchy is broken down into three levels. Level 1 inputs are quoted prices in active markets for identical assets or liabilities. Level 2 inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly. Level 3 inputs are unobservable
63
for the asset or liability and their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable. Level 3 may also include certain investment securities for which there is limited market activity or a decrease in the observability of market pricing for the investments, such that the determination of fair value requires significant judgment or estimation.
The following tables provide information by level for financial assets and liabilities that are measured at fair value on a recurring basis (in thousands):
| Total |
| ||||||||||
carrying | ||||||||||||
value as of | Fair Value Measurements Using | |||||||||||
June 30, | Inputs Considered as | |||||||||||
2022 | Level 1 | Level 2 | Level 3 | |||||||||
| ||||||||||||
Assets |
|
|
|
|
|
|
|
| ||||
Exchange traded securities(1) | $ | | $ | | $ | — | $ | — | ||||
Certificates of deposit(2) |
| |
| |
| — |
| — | ||||
Derivative instruments - cash flow hedges |
| |
| — |
| |
| — | ||||
Total assets | $ | | $ | | $ | | $ | — | ||||
Liabilities |
|
|
|
|
|
|
|
| ||||
Contingent consideration | $ | | $ | — | $ | — | $ | | ||||
Derivative instruments - cash flow hedges |
| |
| — |
| |
| — | ||||
Total liabilities | $ | | $ | — | $ | | $ | | ||||
| Total |
| ||||||||||
carrying | ||||||||||||
value as of | Fair Value Measurements Using | |||||||||||
June 30, | Inputs Considered as | |||||||||||
| 2021 |
| Level 1 |
| Level 2 |
| Level 3 | |||||
Assets |
|
|
|
|
|
|
|
| ||||
Exchange traded securities(1) | $ | | $ | | $ | | $ | — | ||||
Certificates of deposit(2) |
| |
| |
| — |
| — | ||||
Derivative instruments - cash flow hedges |
| |
| — |
| |
| — | ||||
Total assets | $ | | $ | | $ | | $ | — | ||||
Liabilities |
|
|
|
|
|
|
|
| ||||
Contingent consideration | $ | | $ | — | $ | — | $ | | ||||
Derivative instruments - cash flow hedges |
| |
| — |
| |
| — | ||||
Total liabilities | $ | | $ | — | $ | | $ | | ||||
| (1) | Included in available-for-sale investments on the balance sheet. The fair value of the Company’s available-for-sale equity investment in CCXI as of June 30, 2022 and June 30, 2021 was $ |
| (2) | Included in available-for-sale investments on the balance sheet. The certificates of deposit have contractual maturity dates within one year. |
64
Fair value measurements of available for sale securities
Available for sale securities excluding warrants are measured at fair value using quoted market prices in active markets for identical assets and are therefore classified as Level 1 assets. The Company’s warrant to purchase additional shares at a specified future price was valued using a Black-Scholes model with observable inputs in active markets and therefore was classified as a Level 2 asset.
Fair value measurements of derivative instruments
In October 2018, the Company entered into forward starting swaps designated as cash flow hedges on outstanding debt. The forward starting swaps reduce the variability of cash flow payments for the Company by converting the variable interest rate on the Company’s long-term debt described in Note 6 to that of a fixed interest rate. Accordingly, as part of the forward starting swaps, the Company exchanges, at specified intervals, the difference between floating and fixed interest amounts based on an initial $
In May 2021, the Company entered into a new forward starting swap designated as a cash flow hedge on forecasted debt. The forward starting swap reduces the variability of cash flow payments for the Company by converting the variable interest rate on the Company’s forecasted variable interest long-term debt to that of a fixed interest rate. Accordingly, as part of the forward starting swap, the Company exchanges, at specified intervals, the difference between floating and fixed interest amounts based on $
Changes in the fair value of the designated hedged instrument are reported as a component of other comprehensive income and reclassified into interest expense over the corresponding term of the cash flow hedge. The Company reclassified $
The Company reclassified $
Fair value measurements of contingent consideration
The Company has $
65
liabilities for the Asuragen acquisition was $
As of June 30, 2022, the Company's obligation for potential contingent consideration payments related to the Quad and B-Mogen acquisitions were relieved as the revenue thresholds and product milestones were not achieved or there is a remote likelihood of achievement in the timeframe established within the purchase agreements. As the result, the Company reversed an accrual for the fair value of the contingent liabilities at the date of settlement.
During the first quarter of fiscal 2022, the Company made a $
The ultimate settlement of contingent consideration liabilities for the Asuragen acquisition could deviate from current estimates based on the actual results of the financial measures described above. This liability is considered to be a Level 3 financial liability that is re-measured each reporting period. The change in fair value of contingent consideration for these acquisitions is included in general and administrative expense.
The following table presents a reconciliation of the liability measured at fair value on a recurring basis using significant unobservable inputs (Level 3) (in thousands):
June 30, | |||||
2022 | 2021 | ||||
Fair value at the beginning of period | $ | | $ | | |
Purchase price contingent consideration (Note 4) |
| — |
| | |
| ( |
| | ||
Payments |
| ( |
| ( | |
Fair value at the end of period | $ | | $ | | |
The use of different assumptions, applying different judgment to matters that inherently are subjective and changes in future market conditions could result in different estimates of fair value of our securities or contingent consideration, currently and in the future. If market conditions deteriorate, we may incur impairment charges for securities in our investment portfolio.
Fair value measurements of other financial instruments – The following methods and assumptions were used to estimate the fair value of each class of financial instrument for which it is practicable to estimate fair value.
Cash and cash equivalents, certificates of deposit, accounts receivable, and accounts payable – The carrying amounts reported in the consolidated balance sheets approximate fair value because of the short-term nature of these items.
Long-term debt – The carrying amounts reported in the consolidated balance sheets for the amount drawn on our line-of-credit facility and long-term debt approximates fair value because our interest rate is variable and reflects current market rates.
66
Note 6. Debt and Other Financing Arrangements:
On August 1, 2018, the Company entered into a new uncollateralized revolving line-of-credit and term loan governed by a Credit Agreement (the Credit Agreement). The Credit Agreement provides for a revolving credit facility of $
The Credit Agreement matures on August 1, 2023 and contains customary restrictive and financial covenants and customary events of default. At the closing on August 1, 2018 the company borrowed $
Note 7. Leases:
As a lessee, the company leases offices, labs, and manufacturing facilities, as well as vehicles, copiers, and other equipment. The Company determines whether a contract is a lease or contains a lease at inception date. Upon commencement date, operating lease right-of-use assets and liabilities are recognized based on the present value of lease payments over the lease term. The discount rate used to calculate present value is the Company’s incremental borrowing rate or, if available, the rate implicit in the lease. The Company determines the incremental borrowing rate for each lease based primarily on its lease term and the economic environment of the applicable country or region. The Company recognizes operating lease expense on a straight-line basis over the lease term. Further, as part of our adoption of ASC 842, the Company also made the accounting policy elections to not capitalize short term leases (defined as a lease with a lease term that is less than 12 months) and to combine lease and non-lease components for all asset classes in determining the lease payments.
Variable lease payments primarily include payments for non-lease components, such as maintenance costs and payments for non-components such as sales tax. During fiscal year 2022, the Company recognized $
The following table summarizes the balance sheet classification of the Company’s operating leases, amounts of right of use assets and lease liabilities, the weighted average remaining lease term, and the weighted average discount rate for the Company’s operating leases (asset and liability amounts are in thousands):
|
| As of |
| ||||
June 30, |
| ||||||
Balance Sheet Classification | 2022 |
| |||||
Operating leases: |
|
|
|
| |||
Operating lease right of use assets(1) |
| Right of Use Asset | $ | | |||
Current operating lease liabilities(1) |
| Operating lease liabilities current | $ | | |||
Noncurrent operating lease liabilities(1) |
| Operating lease liabilities |
| | |||
Total operating lease liabilities | $ | | |||||
Weighted average remaining lease term (in years): |
|
| |||||
Weighted average discount rate: |
|
| | % | |||
67
| (1) | The right of use asset, current operating lease liabilities, and noncurrent lease liabilities on the Consolidated Balance Sheet exclude a definitive agreement entered into by the Company in November 2021 for a |
The following table summarizes the cash paid for amounts included in the measurement of operating lease liabilities and right of use assets obtained in exchange for new operating lease liabilities for the year ended June 30, 2022 (in thousands):
Year ended | |||
June 30, | |||
| 2022 | ||
Cash amounts paid on operating lease liabilities(1) | $ | | |
Right of use assets obtained in exchange for lease liabilities |
| | |
| (1) | Total cash paid for the Company’s operating leases during the year ended June 30, 2022 include cash amounts paid on operating lease liabilities and variable lease expenses. Cash flow impacts from right of use assets and lease liabilities are presented net on the cash flow statement in changes in other operating activity. |
68
The following table summarizes payments by date for the Company’s operating leases, which is then reconciled to our total lease obligation (in thousands):
| June 30, 2022 | ||
Operating | |||
Leases | |||
2023 | $ | | |
2024 |
| | |
2025 |
| | |
2026 |
| | |
2027 |
| | |
Thereafter |
| | |
Total | $ | | |
Less: Amounts representing interest |
| | |
Total Lease obligations | $ | | |
Certain leases include one or more options to renew, with terms that extend the lease term up to
Note 8. Supplemental Equity and Accumulated Other Comprehensive Income (loss) Information:
Equity
The Company has declared cash dividends per share of $
69
Accumulated Other Comprehensive Income (loss)
Changes in accumulated other comprehensive income (loss) attributable to Bio-Techne, net of tax, are summarized as follows (in thousands):
Unrealized | |||||||||
Gains | Foreign | ||||||||
(Losses) on | Currency | ||||||||
Derivative | Translation | ||||||||
| Instruments |
| Adjustments |
| Total | ||||
Balance June 30, 2019 | $ | ( | $ | ( | $ | ( | |||
Other comprehensive income (loss) before reclassifications |
| ( |
| ( |
| ( | |||
Reclassification from loss on derivatives to interest expense, net of taxes(1) | | — | | ||||||
Balance June 30, 2020(3) | $ | ( | $ | ( | $ | ( | |||
Other comprehensive income (loss) before reclassifications, net of taxes, attributable to Bio-Techne(2) |
| |
| |
| | |||
Reclassification from loss on derivatives to interest expense, net of taxes, attributable to Bio-Techne(1) |
| |
| — |
| | |||
Balance as of June 30, 2021(3) | $ | ( | $ | ( | $ | ( | |||
Other comprehensive income (loss) before reclassifications, net of taxes, attributable to Bio-Techne(2) |
| |
| ( |
| ( | |||
Reclassification from loss on derivatives to interest expense, net of taxes, attributable to Bio-Techne(1) |
| | | ||||||
Balance as of June 30, 2022(3) | $ | | $ | ( | $ | ( | |||
| (1) | Gains (losses) on the interest swap will be reclassified into interest expense as payments on the derivative agreement are made. The Company reclassified $ |
| (2) | Other comprehensive income related to foreign currency translation adjustments in the table above includes the amount attributable to Bio-Techne and excludes the $ |
| (3) | The Company had a net deferred tax liability of $ |
70
Note 9. Earnings Per Share:
The following table reflects the calculation of basic and diluted earnings per share (in thousands, except per share amounts):
Year Ended June 30, | ||||||||
2022 |
| 2021 |
| 2020 | ||||
Earnings per share – basic: | ||||||||
Net earnings, including noncontrolling interest | |
| |
| | |||
Less net earnings (loss) attributable to noncontrolling interest | ( |
| ( |
| — | |||
Net earnings attributable to Bio-Techne | $ | | $ | | $ | | ||
Income allocated to participating securities |
| ( |
| ( |
| ( | ||
Income available to common shareholders | $ | | $ | | $ | | ||
Weighted-average shares outstanding – basic |
| |
| |
| | ||
Earnings per share – basic | $ | | $ | | $ | | ||
| ||||||||
Earnings per share – diluted: |
|
|
|
|
|
| ||
Net earnings, including noncontrolling interest | $ | | $ | | $ | | ||
Less net earnings (loss) attributable to noncontrolling interest | ( | ( | — | |||||
Net earnings attributable to Bio-Techne | | | | |||||
Income allocated to participating securities |
| ( |
| ( |
| ( | ||
Income available to common shareholders | $ | | $ | | $ | | ||
Weighted-average shares outstanding – basic |
| |
| |
| | ||
Dilutive effect of stock options and restricted stock units |
| |
| |
| | ||
Weighted-average common shares outstanding – diluted |
| |
| |
| | ||
Earnings per share – diluted | $ | | $ | | $ | | ||
Basic net income per common share is calculated based on the weighted average number of common shares outstanding during the period. Diluted net income per common share is computed by dividing net income by the weighted average number of common and potentially dilutive common shares outstanding during the period. Potentially dilutive common shares of our stock result from dilutive common stock options and restricted stock units. We use the treasury stock method to calculate the weighted-average shares used in the diluted earnings per share computation. Under the treasury stock method, the proceeds from exercise of an option, the amount of compensation cost, if any, for future service that we have not yet recognized, and the amount of estimated tax benefits that would be recorded in paid-in capital, if any, when the option is exercised are assumed to be used to repurchase shares in the current period.
The dilutive effect of stock options in the above table excludes all options for which the aggregate exercise proceeds exceeded the average market price for the period. The number of potentially dilutive option shares excluded from the calculation was
71
Note 10. Share-based Compensation and Other Benefit Plans:
The cost of employee services received in exchange for the award of equity instruments is based on the fair value of the award at the date of grant. Compensation cost is recognized using a straight-line method over the vesting period and is net of estimated forfeitures. Stock option exercises and stock awards are satisfied through the issuance of new shares.
Equity incentive plan: The 2020 Equity Incentive Plan, which replaced the Company’s Second Amended and Restated 2010 Equity Incentive Plan, provides for the granting of incentive and nonqualified stock options, restricted stock, restricted stock units, performance shares, performance units and stock appreciation rights. There were
The fair values of options granted under the Plans were estimated on the date of grant using the Black-Scholes option-pricing model with the following assumptions used:
Year Ended June 30, | |||||||
| 2022 | 2021 | 2020 | ||||
Dividend yield | % | % | % | ||||
Expected volatility | % | % | % | ||||
Risk-free interest rates | % | % | % | ||||
Expected lives (years) | |||||||
The dividend yield is based on the Company’s historical annual cash dividend divided by the market value of the Company’s common stock. The expected annualized volatility is based on the Company’s historical stock price over a period equivalent to the expected life of the option granted. The risk-free interest rate is based on U.S. Treasury constant maturity interest rates with a term consistent with the expected life of the options granted.
72
Stock option activity under the Plans for the three years ended June 30, 2022, consists of the following (shares in thousands):
|
| Weighted |
| Aggregate |
| Weighted | ||||
Number of | Average | Intrinsic | Average | |||||||
Shares (in | Exercise | Value | Contractual | |||||||
thousands) | Price | (millions) | Life (years) | |||||||
Outstanding at June 30, 2019 |
| | $ | |
|
|
|
| ||
Granted |
| |
| |
|
|
|
| ||
Forfeited |
| ( |
| |
|
|
|
| ||
Exercised |
| ( |
| |
|
|
|
| ||
Outstanding at June 30, 2020 |
| | $ | |
|
|
|
| ||
Granted |
| |
| |
|
|
|
| ||
Forfeited |
| ( |
| |
|
|
|
| ||
Exercised |
| ( |
| |
|
|
|
| ||
Outstanding at June 30, 2021 |
| | $ | |
|
|
|
| ||
Granted |
| |
| |
|
|
|
| ||
Forfeited |
| ( |
| |
|
|
|
| ||
Exercised |
| ( |
| |
|
|
|
| ||
Outstanding at June 30, 2022 |
| | $ | | $ | |
| |||
Exercisable at June 30, 2020: |
| |
| |
|
|
|
| ||
Exercisable at June 30, 2021: |
| |
| |
|
|
|
| ||
Exercisable at June 30, 2022: |
| |
| |
| |
| |||
The weighted average fair value of options granted during fiscal 2022, 2021, and 2020 was $
Restricted common stock activity under the Plans for the three years ended June 30, 2022, consists of the following (units in thousands):
|
|
| Weighted | ||||
Average | |||||||
Weighted | Remaining | ||||||
Number of | Average Grant | Contractual | |||||
Shares (in | Date Fair | Term | |||||
thousands) | Value | (years) | |||||
Unvested at June 30, 2019 |
| | $ | |
|
| |
Granted |
| |
| |
|
| |
Vested |
| ( |
| |
|
| |
Forfeited |
| — |
| — |
|
| |
Unvested at June 30, 2020 |
| | $ | |
|
| |
Granted |
| |
| |
|
| |
Vested |
| ( |
| |
|
| |
Forfeited |
| — |
| — |
|
| |
Unvested at June 30, 2021 |
| | $ | |
|
| |
Granted |
| |
| |
|
| |
Vested |
| ( |
| |
|
| |
Forfeited |
| — |
| — |
|
| |
Unvested at June 30, 2022 |
| | $ | |
The total fair value of restricted shares that vested was $
73
Restricted stock unit activity under the Plans for the three years ended June 30, 2022, consists of the following (units in thousands):
Weighted | |||||||
Average | |||||||
Weighted | Remaining | ||||||
Number of | Average Grant | Contractual | |||||
Units | Date Fair | Term | |||||
| (in thousands) |
| Value |
| (years) | ||
Outstanding at June 30, 2019 |
| |
| $ | |
|
|
Granted |
| |
| |
|
| |
Vested |
| ( |
| |
|
| |
Forfeited |
| ( |
| |
|
| |
Outstanding at June 30, 2020 |
| |
| $ | |
|
|
Granted |
| |
| |
|
| |
Vested |
| ( |
| |
|
| |
Forfeited |
| — |
| — |
|
| |
Outstanding at June 30, 2021 |
| |
| $ | |
|
|
Granted |
| |
| |
|
| |
Vested |
| ( |
| |
|
| |
Forfeited |
| ( |
| |
|
| |
Outstanding at June 30, 2022 |
| |
| $ | |
|
The total fair value of restricted stock units that vested was $
Stock-based compensation cost, inclusive of payroll taxes, of $
Employee stock purchase plan: In fiscal year 2015, the Company established the Bio-Techne Corporation 2014 Employee Stock Purchase Plan (ESPP), which was approved by the Company’s shareholders on October 30, 2014, and which is designed to comply with IRS provisions governing employee stock purchase plans.
Profit sharing and savings plans: The Company has profit sharing and savings plans for its U.S. employees, which conform to IRS provisions for 401(k) plans. The Company makes matching contributions to the Plan. The Company has recorded an expense for contributions to the plans of $
Performance incentive programs: In fiscal 2022, under certain employment agreements, a Management Incentive Plan, and a business incentive plan, available to executive officers, certain management personnel, and certain other professional employees, the Company recorded cash bonuses of $
74
Note 11. Income Taxes:
Income before income taxes was comprised of the following (in thousands):
Year Ended June 30, | |||||||||
| 2022 |
| 2021 |
| 2020 | ||||
Domestic | $ | | $ | | $ | | |||
Foreign |
| |
| |
| | |||
Income before income taxes | $ | | $ | | $ | | |||
The provision for income taxes consisted of the following (in thousands):
Year Ended June 30, | |||||||||
| 2022 |
| 2021 |
| 2020 | ||||
Taxes on income consist of: | |||||||||
Currently tax provision: |
|
|
|
|
|
| |||
Federal |
| $ | |
| $ | |
| $ | |
State |
| |
| |
| | |||
Foreign |
| |
| |
| | |||
Total current tax provision |
| |
| |
| | |||
Deferred tax provision: |
|
|
|
|
|
| |||
Federal |
| |
| ( |
| | |||
State |
| |
| ( |
| | |||
Foreign |
| ( |
| ( |
| ( | |||
Total deferred tax provision |
| |
| ( |
| | |||
Total income tax provision |
| $ | |
| $ | |
| $ | |
The Company’s effective income tax rate for fiscal 2022 was
The Company’s effective income tax rate for fiscal 2021 was
The Company’s discrete tax benefits in fiscal 2022, 2021, and 2020 primarily related to share-based compensation excess tax benefits of $
75
The following is a reconciliation of the federal tax calculated at the statutory rate of to the actual income taxes provided:
| Year Ended June 30, | |||||||||
| 2022 |
| 2021 |
| 2020 | |||||
Income tax expense at federal statutory rate | | % |
| | % |
| | % | ||
State income taxes, net of federal benefit | |
| |
| | |||||
Research and development tax credit | ( |
| ( |
| ( | |||||
Contingent consideration adjustment | ( |
| |
| ( | |||||
Foreign tax rate differences | |
| |
| ( | |||||
Impairment | | — | — | |||||||
Option exercises | ( |
| ( |
| ( | |||||
U.S. taxation of foreign earnings | ( |
| ( |
| | |||||
Foreign derived intangible income | ( |
| ( |
| ( | |||||
Executive compensation limitations | |
| |
| | |||||
Other, net | ( |
| |
| ( | |||||
Effective tax rate | | % |
| | % |
| | % | ||
Deferred taxes on the Consolidated Balance Sheets consisted of the following temporary differences (in thousands):
June 30, | ||||||
| 2022 |
| 2021 | |||
Inventory | $ | | $ | | ||
Net operating loss carryovers |
| |
| | ||
Tax credit carryovers |
| |
| | ||
Excess tax basis in equity investments |
| |
| | ||
Deferred compensation |
| |
| | ||
Derivative - cash flow hedge |
| — |
| | ||
Lease liability |
| |
| | ||
Other |
| |
| | ||
Valuation allowance |
| ( |
| ( | ||
Deferred tax assets |
| |
| | ||
Net unrealized gain on available-for-sale investments |
| ( |
| ( | ||
Intangible asset amortization |
| ( |
| ( | ||
Depreciation |
| ( |
| ( | ||
Right of use asset |
| ( |
| ( | ||
Derivative - cash flow hedge | ( | — | ||||
Other |
| ( |
| ( | ||
Deferred tax liabilities |
| ( |
| ( | ||
Net deferred tax liabilities | $ | ( | $ | ( | ||
A deferred tax valuation allowance is required when it is more likely than not that all or a portion of deferred tax assets will not be realized. The valuation allowance as of June 30, 2022 was $
As of June 30, 2022, the $
As of June 30, 2022, the Company has federal operating loss carryforwards of approximately $
76
Section 382. As of June 30, 2022, the Company has foreign net operating loss carryforwards of $
As of June 30, 2022, the Company has approximately $
We continue to analyze our global working capital requirements and the potential tax liabilities that would be incurred if the non-U.S. subsidiaries distribute cash to the U.S. parent, which include local country withholding tax and potential U.S. state taxation.
The following is a reconciliation of the beginning and ending balance of unrecognized tax benefits (in thousands):
Year Ended June 30, | |||||||||
| 2022 |
| 2021 |
| 2020 | ||||
Beginning balance | $ | | $ | | $ | | |||
Additions due to acquisitions |
| |
| — |
| — | |||
Additions for tax positions of prior year |
| |
|
| |
| | ||
Decrease in unrecognized tax benefits for prior year positions |
| ( |
|
| ( |
| ( | ||
Settlements |
| ( |
|
| ( |
| — | ||
FX impact | ( | — | — | ||||||
Ending balances | $ | | $ | | $ | | |||
Included in the balance of unrecognized tax benefits at June 30, 2022 are potential benefits of $
Note 12. Segment Information:
The Company operates under
The Company’s Protein Sciences segment is comprised of the reagent solutions and analytical solutions. These businesses manufacture consumables used for conducting laboratory experiments by both industry and academic scientists within the biotechnology and biomedical life science fields. No customer in the Protein Sciences segment accounted for more than 10% of the segment’s net sales for the years ended June 30, 2022, 2021, and 2020.
77
The Company’s Diagnostics and Genomics segment is comprised of diagnostics reagents, genomics, and molecular diagnostics, which includes our Exosome and Asuragen acquisitions. Diagnostics reagents develops and manufactures a range of controls and calibrators used with diagnostic equipment and as proficiency testing tools, as well as other reagents incorporated into diagnostic kits. Genomics and molecular diagnostics consists of exosome-based diagnostics products for various pathologies, as well as tissue-based in-situ hybridization assays for research in clinical use. No customer in the Diagnostics and Genomics segment accounted for more than 10% of the segment’s net sales for the fiscal years ended June 30, 2022, 2021, and 2020.
There are no concentrations of business transacted with a particular customer or supplier or concentrations of revenue from a particular product or geographic area that would severely impact the Company in the near term.
Following is financial information relating to the operating segments (in thousands):
Year Ended June 30, | ||||||||
2022 |
| 2021 |
| 2020 | ||||
Net sales: |
| |||||||
Protein Sciences | $ | |
| $ | | $ | | |
Diagnostics and Genomics |
| |
| |
| | ||
Intersegment |
| ( |
| ( |
| ( | ||
Consolidated net sales | $ | |
| $ | | $ | | |
Operating income: |
|
|
|
|
|
| ||
Protein Sciences(1) | $ | |
| $ | | $ | | |
Diagnostics and Genomics |
| |
| |
| | ||
Segment operating income |
| |
| |
| | ||
Costs recognized on sale of acquired inventory |
| ( |
| ( |
| — | ||
Amortization of acquisition related intangible assets |
| ( |
| ( |
| ( | ||
Impact of partially owned consolidated subsidiaries(1) |
| ( |
| ( |
| — | ||
Gain on escrow settlement | — | — | | |||||
Acquisition related expenses |
| |
| ( |
| ( | ||
Eminence impairment | ( | — | — | |||||
Stock based compensation, inclusive of employer taxes |
| ( |
| ( |
| ( | ||
Restructuring costs |
| ( |
| ( |
| ( | ||
Corporate general, selling, and administrative expenses(1) |
| ( |
| ( |
| ( | ||
Consolidated operating income | $ | |
| $ | | $ | | |
(1)Adjusted operating income for fiscal 2021 have been updated for comparability to fiscal 2022 for the inclusion of the impact of partially-owned consolidated subsidiaries on the Company’s adjusted operating income.
The Company has some integrated facilities that serve both segments. As such, asset and capital expenditure information by operating segment has not been provided and is not available, since the Company does not produce or utilize such information internally. In addition, although depreciation and amortization expense is a component of each operating segment’s operating results, it is not discretely identifiable.
78
The Company has disclosed sales by geographic area based on the location of the customer or distributor in Note 2. The Company has disclosed dis-aggregated product and service revenue by consumables, instruments, and services in Note 2. The Company considers total instrument and total service revenue to represent similar groups of products in the fiscal years presented. The Company considered our consumables sold in the Protein Sciences and Diagnostics and Genomics segments to represent different groups of products and therefore have separately disclosed the related consumables revenue (in thousands) :
Year Ended June 30, | |||||||||
| 2022 |
| 2021 |
| 2020 | ||||
Consumables revenue - Protein Sciences | $ | | $ | | $ | | |||
Consumables revenue - Diagnostics and Genomics |
| |
| |
| | |||
Total consumable revenue | $ | | $ | | $ | | |||
The following is financial information relating to geographic areas (in thousands):
Year ended June 30, | ||||||
| 2022 |
| 2021 | |||
Long-lived assets: | ||||||
United States and Canada | $ | |
| $ | | |
Europe |
|
| |
| | |
Asia |
| |
| | ||
Total long-lived assets | $ | |
| $ | | |
Intangible assets: |
|
|
|
| ||
United States and Canada | $ | |
| $ | | |
Europe |
| |
| | ||
Asia |
| |
| | ||
Total intangible assets | $ | |
| $ | | |
Long-lived assets are comprised of land, buildings and improvements and equipment, net of accumulated depreciation.
Note 13. Subsequent Events:
On July 1, 2022, the Company completed the acquisition of Namocell, Inc. for approximately $
On August 4, 2022, the Company sold its remaining shares in CCXI for $
79
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Bio-Techne Corporation:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Bio-Techne Corporation and subsidiaries (the Company) as of June 30, 2022 and 2021, the related consolidated statements of earnings and comprehensive income, shareholders’ equity, and cash flows for each of the years in the three-year period ended June 30, 2022, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the three-year period ended June 30, 2022, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of June 30, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated August 24, 2022 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of a critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Goodwill impairment analysis for the Molecular Diagnostics Division reporting unit
As discussed in Note 1 to the consolidated financial statements, the goodwill balance as of June 30, 2022 was $822.1 million, of which $195.9 million related to the Molecular Diagnostics Division (MDD) reporting unit. The Company performs goodwill impairment testing on an annual basis and whenever events or changes in circumstances indicate that the carrying value of a reporting unit likely exceeds its fair value. This involves estimating the fair value of the reporting units using discounted cash flow models.
80
We identified the evaluation of the goodwill impairment analysis for the MDD reporting unit as a critical audit matter. There was a high degree of subjectivity in applying and evaluating certain key assumptions used in the discounted cash flow model to estimate the fair value of the MDD reporting unit. Specifically, the revenue growth rates and the discount rate were challenging to test as they represented subjective determinations of future market and economic conditions. Changes to those assumptions could have had a significant effect on the Company’s assessment of the fair value of the MDD reporting unit.
The following are the primary procedures we performed to address this critical audit matter. We evaluated the design and tested the operating effectiveness of certain internal controls related to the goodwill impairment process. This included controls related to the Company’s determination of the estimated fair value of the MDD reporting unit, including controls related to the development of the assumptions for the revenue growth rates and discount rate. We performed sensitivity analyses over the revenue growth rates and discount rate assumptions to assess their impact on the Company’s determination that the fair value of the MDD reporting unit exceeded its carrying value. We evaluated the reasonableness of the Company’s forecasted revenue growth rates for the MDD reporting unit by comparing the growth rate assumptions to historical results and industry related third-party data. We also compared the Company’s historical revenue forecasts to actual results to assess the Company’s ability to accurately forecast. In addition, we involved valuation professionals with specialized skills and knowledge, who assisted in evaluating the discount rate used in the valuation, by comparing it against a discount rate range that was independently developed using publicly available market data for comparable entities.
/s/
We have served as the Company’s auditor since 2002.
August 24, 2022 |
81
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Bio-Techne Corporation:
Opinion on Internal Control Over Financial Reporting
We have audited Bio-Techne Corporation and subsidiaries' (the Company) internal control over financial reporting as of June 30, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of June 30, 2022, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of June 30, 2022 and June 30, 2021, the related consolidated statements of earnings and comprehensive income, shareholders’ equity, and cash flows for each of the years in the three-year period ended June 30, 2022, and the related notes (collectively, the consolidated financial statements), and our report dated August 24, 2022 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Minneapolis, Minnesota | |
August 24, 2022 |
82
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
(a) | Evaluation of Disclosure Controls and Procedures |
As required by Rule 13a-15(b) of the Securities Exchange Act of 1934 (the "Exchange Act"), management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated, as of the end of the period covered by this report, the effectiveness of our disclosure controls and procedures as defined in Exchange Act Rule 13a-15(e). The evaluation was based upon reports and certifications provided by a number of executives. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of June 30, 2022, our disclosure controls and procedures were effective.
(b) | Management’s Annual Report on Internal Control Over Financial Reporting |
The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting also includes those policies and procedures that:
(i) | Pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
(ii) | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
(iii) | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
Under the supervision of the Audit Committee of the Board of Directors and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting using the criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on our assessment and those criteria, our Chief Executive Officer and Chief Financial Officer concluded that our internal control over financial reporting was effective as of June 30, 2022.
The attestation report on our internal control over financial reporting issued by KPMG LLP appears in Item 8 of this report.
(c) | Changes in Internal Control Over Financial Reporting |
83
As previously announced, we acquired Changzhou Eminence Biotechnology Co., Ltd on October 20, 2020, and Asuragen, Inc. on April 6, 2021 and we have implemented our internal control structure over these and incorporated their operations into our assessment of internal control over financial reporting as of June 30, 2022.
There were no other changes in the Company’s internal control over financial reporting during fiscal year 2022 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
84
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Other than "Executive Officers of the Registrant" which is set forth at the end of Item 1 in Part I of this report, the information required by Item 10 is incorporated herein by reference to the sections entitled "Election of Directors," "Principle Shareholders" and "Additional Corporate Governance Matters" in the Company’s Proxy Statement for its 2021 Annual Meeting of Shareholders which will be filed with the Securities and Exchange Commission pursuant to Regulation 14A within 120 days after the close of the fiscal year for which this report is filed.
ITEM 11. EXECUTIVE COMPENSATION
The information required by Item 11 is incorporated herein by reference to the sections entitled "Election of Directors" and "Executive Compensation" in the Company’s Proxy Statement for its 2022 Annual Meeting of Shareholders which will be filed with the Securities and Exchange Commission pursuant to Regulation 14A within 120 days after the close of the fiscal year for which this report is filed.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED SHAREHOLDER MATTERS
The information required by Item 12 is incorporated by reference to the sections entitled "Principal Shareholders" and "Management Shareholdings" in the Company’s Proxy Statement for its 2022 Annual Meeting of Shareholders which will be filed with the Securities and Exchange Commission pursuant to Regulation 14A within 120 days after the close of the fiscal year for which this report is filed.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by Item 13 is incorporated by reference to the sections entitled "Election of Directors" and "Additional Corporate Governance Matters" in the Company’s Proxy Statement for its 2022 Annual Meeting of Shareholders which will be filed with the Securities and Exchange Commission pursuant to Regulation 14A within 120 days after the close of the fiscal year for which this report is filed.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by Item 14 is incorporated herein by reference to the section entitled "Audit Matters" in the Company’s Proxy Statement for its 2022 Annual Meeting of Shareholders which will be filed with the Securities and Exchange Commission pursuant to Regulation 14A within 120 days after the close of the fiscal year for which this report is filed.
85
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
A. (1) List of Financial Statements.
The following Consolidated Financial Statements are filed as part of this Annual Report on Form 10-K:
Consolidated Statements of Earnings and Comprehensive Income for the Years Ended June 30, 2022, 2021, and 2020
Consolidated Balance Sheets as of June 30, 2022 and 2021
Consolidated Statements of Shareholders’ Equity for the Years Ended June 30, 2022, 2021, and 2020
Consolidated Statements of Cash Flows for the Years Ended June 30, 2022, 2021, and 2020
Notes to Consolidated Financial Statements for the Years Ended June 30, 2022, 2021, and 2020
Reports of Independent Registered Public Accounting Firm (PCAOB ID:
A. (2) Financial Statement Schedules.
All financial statement schedules are omitted because they are not applicable, not material or the required information is shown in the Consolidated Financial Statements or Notes thereto.
86
A. (3) Exhibits.
EXHIBIT INDEX
for Form 10-K for the 2022 Fiscal Year
Exhibit Number |
| Description | |
3.1 | |||
|
| ||
3.2 | |||
|
| ||
4.1 | Description of Capital Stock -- attached as Exhibit 4.1 hereto | ||
|
| ||
10.1** | |||
|
| ||
10.2** | |||
|
| ||
10.3** | |||
|
| ||
10.4** | |||
|
| ||
10.5** | |||
|
| ||
10.6** | |||
|
| ||
10.7** | |||
|
| ||
10.8** | |||
10.9** | |||
|
| ||
10.10** | |||
|
| ||
10.11** | |||
|
| ||
10.12** | |||
|
| ||
87
10.13** | |||
10.14** | |||
|
| ||
10.15** | |||
|
| ||
10.16 | |||
|
| ||
10.17** | |||
10.18** |
| ||
10.20 | |||
10.30** | |||
10.40** | |||
10.50** | |||
10.60** | |||
10.70** | |||
10.80** | |||
10.90** | |||
20** | |||
20.1** | |||
21 |
| ||
23 | Consent of KPMG LLP, Independent Registered Public Accounting Firm | ||
88
|
| ||
31.1 | Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | ||
|
| ||
31.2 | Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | ||
|
| ||
32.1 | Certification of Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | ||
|
| ||
32.2 | Certification of Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | ||
|
| ||
101 | The following financial statements from the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2022, formatted in Inline Extensible Business Reporting Language (iXBRL): (i) the Consolidated Statements of Earnings and Comprehensive Income, (ii) the Consolidated Balance Sheets, (iii) the Consolidated Statements of Shareholders’ Equity, (iv) the Consolidated Statements of Cash Flows, and (v) Notes to the Consolidated Financial Statements. | ||
|
| ||
104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | ||
* | Incorporated by reference; SEC File No. 000-17272 |
** | Management contract or compensatory plan or arrangement |
Exhibits for Form 10-K have not been included in this report. Exhibits have been filed with the Securities and Exchange Commission. Upon request to the Investor Relations Department, Bio-Techne Corporation will furnish, without charge, any such exhibits as well as copies of periodic reports filed with the Securities and Exchange Commission
ITEM 16. FORM 10-K SUMMARY
None.
89
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| BIO-TECHNE CORPORATION | |||
|
|
| ||
Date: August 24, 2022 | /s/ Charles Kummeth | |||
|
| By: | Charles Kummeth | |
Its: | President and CEO | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Date | Signature and Title | |
|
| |
August 24, 2022 | /s/ Robert V. Baumgartner | |
| Robert V. Baumgartner | |
| Chairman of the Board and Director | |
|
| |
August 24, 2022 | /s/ Julie Bushman | |
| Julie Bushman, Director | |
|
| |
August 24, 2022 | /s/ Rupert Vessey | |
| Dr. Rupert Vessey, Director | |
|
| |
August 24, 2022 | /s/ Joseph Keegan, Ph.D. | |
| Dr. Joseph Keegan, Director | |
|
| |
August 24, 2022 | /s/ John L. Higgins | |
| John L. Higgins, Director | |
|
| |
August 24, 2022 | /s/ Roeland Nusse, Ph.D. | |
| Dr. Roeland Nusse, Director | |
|
| |
August 24, 2022 | /s/ Alpna Seth, Ph.D. | |
| Dr. Alpna Seth, Director | |
|
| |
August 24, 2022 | /s/ Randolph C. Steer, Ph.D., M.D. | |
| Dr. Randolph C. Steer, Director | |
|
| |
August 24, 2022 | /s/ Charles Kummeth | |
| Charles Kummeth, Director and Chief Executive Officer (principal executive officer) | |
|
| |
August 24, 2022 | /s/ James Hippel | |
| James Hippel, Chief Financial Officer | |
| (principal financial officer and principal accounting officer) |
90