Bio-Techne Scales Antibody Validation Initiative Using CRISPR Gene Editing Technology Across a Broad Spectrum of Targets
Knockout Validation of Antibodies Confirms Specificity and Ensures Reproducible Results
MINNEAPOLIS, July 10, 2018 /PRNewswire/ -- Bio-Techne Corporation (NASDAQ: TECH), a family of brands with a unique and leading portfolio of antibody-based reagents for life science and clinical research, has been validating an extensive and growing number of antibodies using recent advances in gene editing technology.
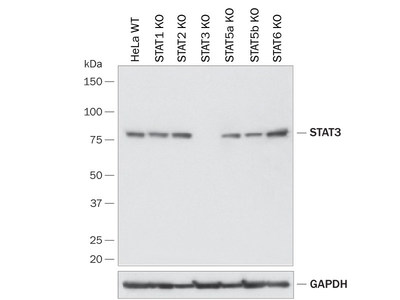
The specificity and power of the CRISPR gene editing system is now being utilized by Bio-Techne for the validation of antibodies in knockout (KO) cell lines. This methodology is one of the proposed, and currently most effective, negative controls identified by the International Working Group for Antibody Validation (Uhlen et al., Nat. Methods, 2016). Bio-Techne has now validated R&D Systems- and Novus Biologicals-branded antibodies for over 110 different targets from a wide range of CRISPR KO-modified cell lines and partnered with two gene-editing companies, B-MoGen Biotechnologies and EdiGENE Corporation, to produce biologically relevant data for nearly 1,000 antibody products.
The KO antibody initiative covers a broad spectrum of targets from receptors, enzymes, transcription factors, and autophagy markers to immune checkpoint molecules. Bio-Techne gene knockouts are produced in over 10 different cell line models, all of which have been carefully selected to include those frequently used by scientists in their daily research, making these lines the most relevant in vitro model. Additionally, Bio-Techne performs antibody validation for various applications using KO cell lines, including Western blot, immunocytochemistry, and flow cytometry.
Bio-Techne is proud to now make over 1,750 KO cell lysates for Western blots available to scientists for performing antibody validation in their own laboratories. In addition to our continuously growing KO validation initiative, thousands of our affinity reagents have been validated across multiple biological models, verified against our exhaustive library of recombinant proteins and transfected cell lines, compared to independent antibody clones, and tested using other orthogonal methods.
Dave Eansor, Senior Vice President of the Biotech Division at Bio-Techne, commented: "We are proud to make available to researchers even more antibodies validated using CRISPR KO cell lines, including our proprietary recombinant monoclonal antibodies. Bio-Techne's KO validation initiative meets the need from the life science research community for antibodies with enhanced specificity testing. Furthermore, laboratories will also be able to utilize KO cell line lysates as a true negative control in Western blot experiments for internal antibody validation."
For more information about Bio-Techne antibody products, please visit: www.novusbio.com or www.rndsystems.com

About Bio-Techne Corporation (NASDAQ: TECH)
Contact: Dave Eansor, Senior Vice President, Biotech Division, 612-379-2956

![]() View original content with multimedia:http://www.prnewswire.com/news-releases/bio-techne-scales-antibody-validation-initiative-using-crispr-gene-editing-technology-across-a-broad-spectrum-of-targets-300677910.html
View original content with multimedia:http://www.prnewswire.com/news-releases/bio-techne-scales-antibody-validation-initiative-using-crispr-gene-editing-technology-across-a-broad-spectrum-of-targets-300677910.html
SOURCE Bio-Techne Corporation
Released July 10, 2018